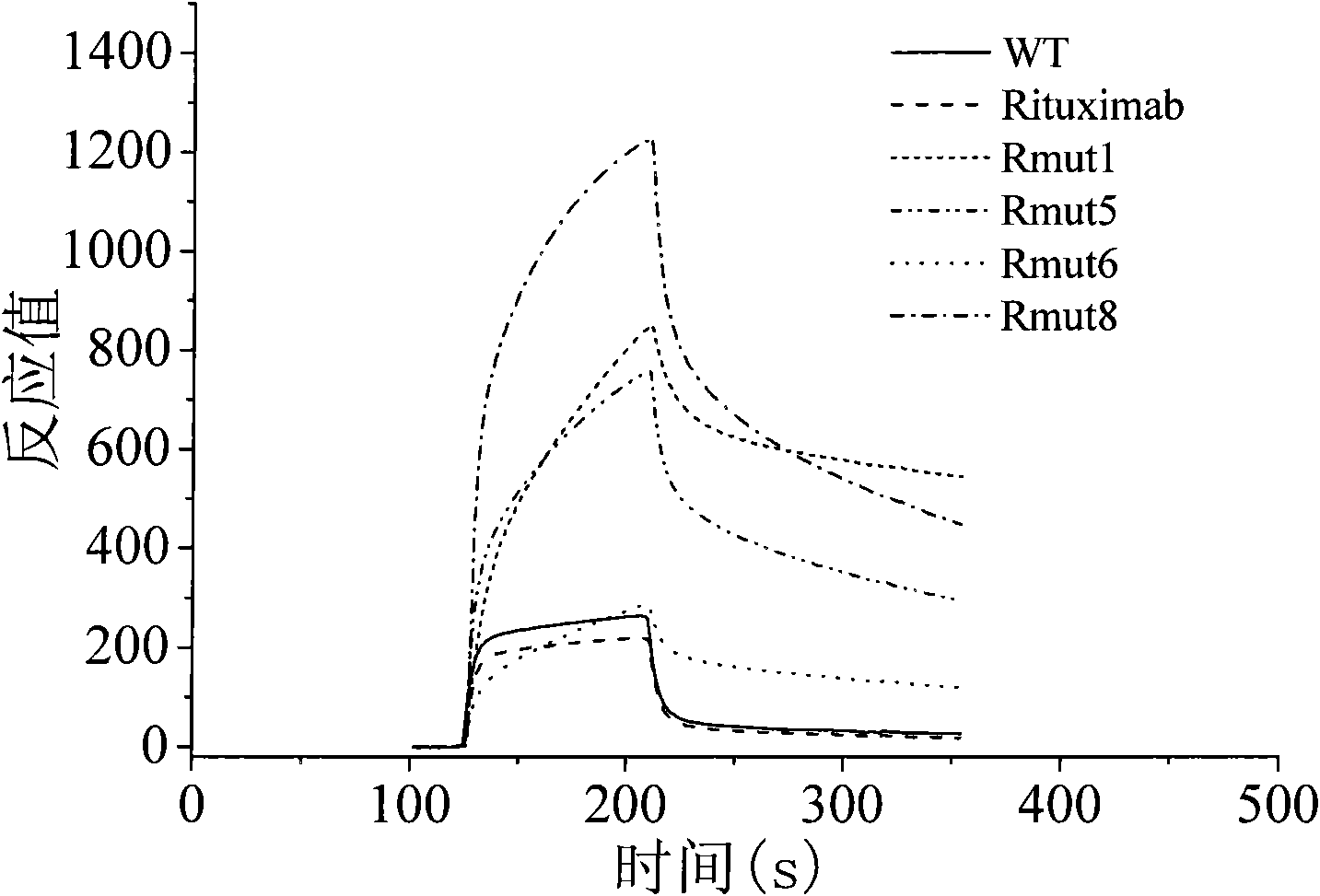
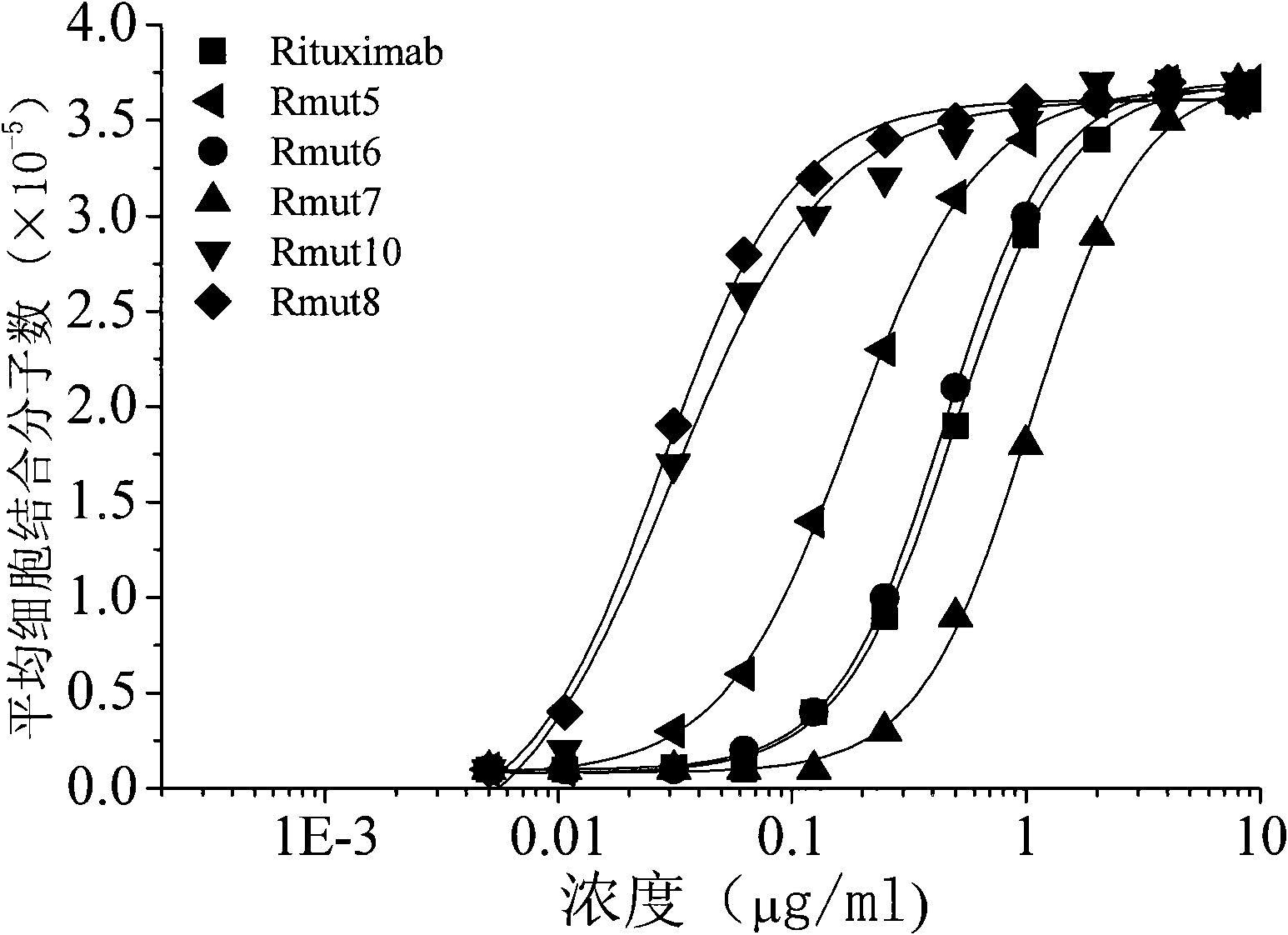
High-affinity CD20-resistance monoclonal antibody
A monoclonal antibody, affinity technology, applied in the field of genetic engineering products, can solve the problems of reduced curative effect, easy recurrence or drug resistance, difficult to cure, etc., and achieve obvious anti-tumor effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Cloning of human antibody light and heavy chain constant region genes
[0040]Healthy human lymphocytes were isolated with lymphocyte separation solution (product of Dingguo Biotechnology Development Co., Ltd.), and total RNA was extracted with Trizol reagent (product of Invitrogen company). According to literature (Cloned human and mouse kappaimmunoglobulin constant and J region genes conserve homology in functional segments) . Hieter PA, Max EE, Seidman JG, Maizel JV Jr, Leder P. Cell. 1980 Nov; 22(1 Pt 1): 197-207.) and literature (The nucleotide sequence of a human immunoglobulin C gamma1 gene. Ellison JW, Berson BJ, Hood LE. Nucleic Acids Res. 1982 Jul 10; 10(13): 4071-9.) designed primers respectively to amplify antibody heavy chain and light chain constant region genes by RT-PCR reaction. The PCR product was purified and recovered by agarose gel electrophoresis and cloned into pGEM-T vector (promega company). After sequencing and verification, it was c...
Embodiment 2
[0041] Example 2. Construction of expression vector of anti-CD20 chimeric antibody C2B8
[0042] Referring to the anti-CD20 monoclonal antibody data and sequence disclosed in US Patent 6,399,061, Shanghai Sangon Bioengineering Co., Ltd. was entrusted to fully synthesize the anti-CD20 monoclonal antibody C2B8 heavy chain variable region gene (C2B8VH) and light chain variable region gene (C2B8VL) . figure 1 The nucleotide and amino acid sequences of the C2B8 heavy and light chain variable regions are shown.
[0043] The chimeric antibody heavy chain gene was synthesized by overlapping PCR using the C2B8VH gene and pGEM-T / CH vector as templates. The reaction conditions were: 95°C for 15 minutes; 94°C for 50 seconds, 58°C for 50 seconds, and 72°C for 50 seconds, 30 cycles ; 72°C for 10 minutes. The 5' end of the chimeric antibody heavy chain gene contains the restriction enzyme site HindIII and the signal peptide gene sequence, and the 3' end contains the translation stop codon ...
Embodiment 3
[0045] Example 3. Stable expression and purification of chimeric antibodies
[0046] Inoculate 3×10 in a 3.5cm tissue culture dish 5 CHO-K1 cells (ATCC CRL-9618), transfection when the cells are cultured to 90%-95% confluence: take 10 μg of plasmid (plasmid pcDNA3.1(+)(C2B8VHCH) 4 μg, plasmid pcDNA3.1(C2B8VLCL) 6 μg) and 20 μl Lipofectamine2000 Reagent (product of Invitrogen) were dissolved in 500 μl serum-free DMEM medium, left at room temperature for 5 minutes, the above two liquids were mixed, and incubated at room temperature for 20 minutes to form DNA-liposome complexes. Serum-free DMEM medium replaces the serum-containing medium in the dish, then the formed DNA-liposome complexes are added to the plate, CO 2 After 4 hours in the incubator, add 2 ml of DMEM complete medium containing 10% serum, and place in CO. 2 continue to grow in the incubator. After 24 hours of transfection, the cells were changed to selection medium containing 600 μg / ml G418 to select resistant cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com