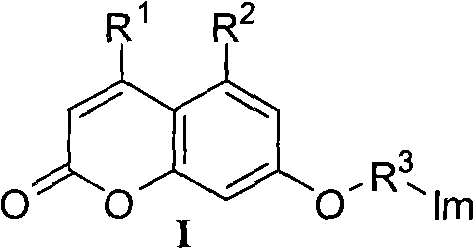
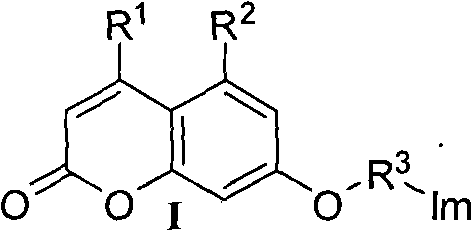
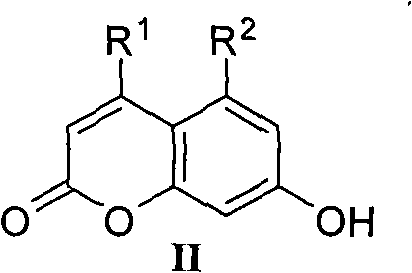
Coumarin azole compound with antimicrobial activity, and preparation method and medicinal application thereof
A technology of coumarin azoles and compounds, applied in the direction of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of drug resistance, toxic and side effects, etc., and achieve low cost, simple synthesis method, and raw materials Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of 7-(2-(1H-1,2,4-triazol-1-yl)-ethoxy)-4-methyl-coumarin (compound 1 for short)
[0032] In a 100mL single-neck round bottom flask, add acetonitrile 30mL, anhydrous potassium carbonate 4.141g (30.0mmol), 1,2,4-triazole 1.321g (20.0mmol), stir at room temperature for 1 hour, add 7-(2- Bromo-ethoxy)-4-methylcoumarin 2.820g (10.0mmol), traced by thin-layer chromatography until the reaction was completed, acetonitrile was removed by distillation under reduced pressure, the residual solid was added with 100mL of water, extracted three times with equal volume of chloroform, and the organic layers were combined. The organic layer was back-extracted once with saturated brine, and the organic layer was dried over anhydrous sodium sulfate. Concentrate the organic phase, and perform column chromatography (using chloroform and acetone (3 / 1, V / V) as eluent) to obtain 2.093 g of white solid, yield: 77.2%; melting point: 199-201 °C; 1 H NMR (400MHz, CDCl 3)δ:...
Embodiment 2
[0033] Example 2: Preparation of 7-(3-(1H-1,2,4-triazol-1-yl)-propoxy)-4-methyl-coumarin (compound 2 for short)
[0034] According to embodiment 1 synthetic method. Starting materials acetonitrile 30mL, anhydrous potassium carbonate 4.140g (30.0mmol), 1,2,4-triazole 1.321g (20.0mmol), 7-(3-bromo-propoxy)-4-methylcoumarin Sodium 2.960g (10.0mmol), 2.221g of white solid was obtained, yield: 77.9%; melting point: 145~146°C; 1 HNMR (400MHz, CDCl 3 )δ: 8.09 (s, 1H, triazole 3-H), 7.97 (s, 1H, triazole 5-H), 7.50~7.48 (d, 1H, coumarin 5-H), 6.83~6.78 (m, 2H, coumarin 6, 8-H), 6.14 (s, 1H, coumarin 3-H), 4.44~4.42 (m, 2H, coumarin-OCH 2 ), 4.00~3.99 (m, 2H, triazole-CH 2 ), 2.43~2.40 (m, 5H, Ar-CH 3 , triazole-CH 2 CH 2 ) ppm.
Embodiment 3
[0035] Example 3: Preparation of 7-(4-(1H-1,2,4-triazol-1-yl)-butoxy)-4-methyl-coumarin (compound 3 for short)
[0036] According to embodiment 1 synthetic method. The starting materials are 30mL of acetonitrile, 4.141g (30.0mmol) of anhydrous potassium carbonate, 1.321g (20.0mmol) of 1,2,4-triazole, 7-(4-bromo-butoxy)-4-methyl aromatic Soybean 3.101g (10.0mmol), 2.300g white solid was obtained, yield: 76.9%; melting point: 85-86°C; 1 H NMR (400MHz, CDCl 3 )δ: 8.13 (s, 1H, triazole 3-H), 7.96 (s, 1H, triazole 5-H), 7.50~7.48 (d, 1H, coumarin 5-H), 6.84~6.78 (m, 2H, coumarin 6, 8-H), 6.13 (s, 1H, coumarin 3-H), 4.28~4.26 (m, 2H, coumarin-OCH 2 ), 4.04~4.03 (m, 2H, triazole-CH 2 ), 2.39 (s, 1H, Ar-CH 3 ), 2.14~2.11 (m, 4H, coumarin-OCH 2 CH 2 , triazole-CH 2 CH 2 ) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com