Immunoassay reagent for assaying influenza A H1N1 virus antigen
An immunodetection, type A technology, applied in antiviral immunoglobulins, antiviral agents, and serum immunoglobulins, etc., can solve the problems of undetectable and low virus content, and achieve high affinity, good specificity, The effect of increasing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] [Example 1] Preparation of DNA vaccine
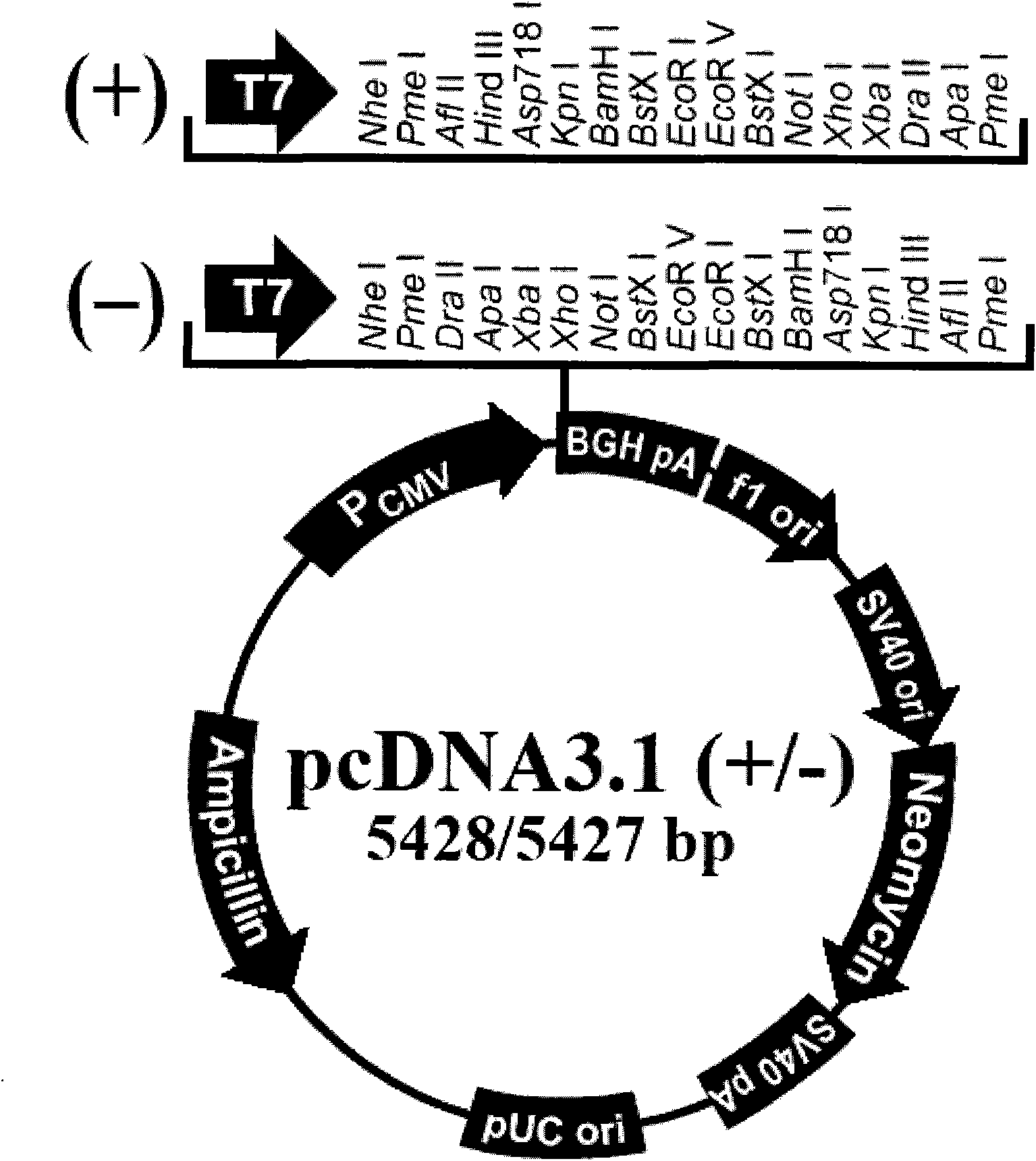
[0023] The HA gene sequence of H1N1 influenza virus A / California / 04 / 2009 is shown in SEQ ID NO.1. The HA sequence was synthesized by Shanghai Sangong and inserted into the multiple cloning site of pCDNA3.1(+) vector after double enzyme digestion (XhoI / ApaI) Point, constructed into pCDNA3.1 / SF-HA vector.
[0024] Large-scale preparation of plasmid DNA
[0025] According to the steps of the QIAGEN Plasmid Maxi Kit produced by QIAGEN Company, the recombinant plasmid DNA was extracted, the gained DNA was dissolved in TE solution, and the OD260 value and OD280 value of the prepared plasmid DNA were measured on a UV spectrophotometer, according to the calculated DNA purity and The concentration formula calculates the purity and concentration of DNA. According to its initial concentration, it was diluted to a final concentration of 1 μg / μl for electrophoresis identification, and the rest were stored in a -20°C refrigerator for later...
Embodiment 2
[0026] [Example 2] Preparation of mouse anti-H1N1 virus antigen monoclonal antibody
[0027] 1. Immunization Methods and Procedures :
[0028] The immunization route adopts the intramuscular injection method of the quadriceps femoris. The amount of DNA injected into each 6-8-week-old BALB / c mouse is 50 μg. , the electric shock time is 50ms), and a booster immunization was carried out 3 weeks after the initial immunization.
[0029] 2. Monoclonal antibody preparation:
[0030] 2.1 Cell Fusion:
[0031] 2.1.1 The mice were killed, and the spleen was removed under aseptic operation. The spleen was placed in a 5ML RPMI-1640 culture dish (without adding serum), cut into two halves, and then the cells were squeezed out with sterilized equipment.
[0032] 2.1.2 Aspirate the cells into a sterile plastic tube, let it stand for 5 minutes to allow the tissue pieces to settle down, transfer the cells to another sterile test tube and centrifuge at 300XG for 10 minutes.
[0033] 2.1...
Embodiment 3
[0045] [Example 3] Preparation of rabbit anti-H1N1 virus antigen polyclonal antibody
[0046] 1. Rabbit polyclonal antibody preparation: Rabbits were immunized by intramuscular injection of 3-month-old rabbits into the quadriceps femoris muscle. The amount of DNA injected was 100 μg. After injection, 3 times of positive and negative electric shocks (100V Voltage, electric shock time is 50ms), and a booster immunization was carried out 3 weeks after the initial immunization. Ten days after the last booster, the rabbits were killed and bled, and the separated serum was stored at -20°C.
[0047] 2. Purification of rabbit polyclonal antibody: rough extraction by salting out method, and then purified by DEAE-cellulose ion exchange chromatography. The purity of the antibody is identified by SDS-PAGE. It is required that two clear bands appear at 25KD and 50KD of the loaded protein, and the purity is above 90%.
[0048] 3. Rabbit polyantibody biotin labeling: Biotin was purchased f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com