Magnesium ion-containing nonaqueous electrolyte solution and electrochemical device using the same
A non-aqueous electrolyte and magnesium ion technology, which is applied in electrochemical generators, battery electrodes, electrode carriers/collectors, etc., can solve the problem of low equilibrium potential and achieve the effect of increasing discharge capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0145] In this embodiment mode, a description will now be given about a nonaqueous electrolytic solution containing magnesium ions therein based on the present invention, and a magnesium battery as an example of an electrochemical device using the nonaqueous electrolytic solution. In this regard, it should be noted in advance that the description given here is only an example, and thus the present invention is by no means limited thereto. Although in the following, a coin type (also called a button type) battery will be described, the present invention can also be applied to a cylindrical or square battery having a spiral structure inside, with a thin positive electrode and a thin negative electrode wound with a separator in between. Into a spiral shape, so the same effect as a coin-type battery can be obtained.
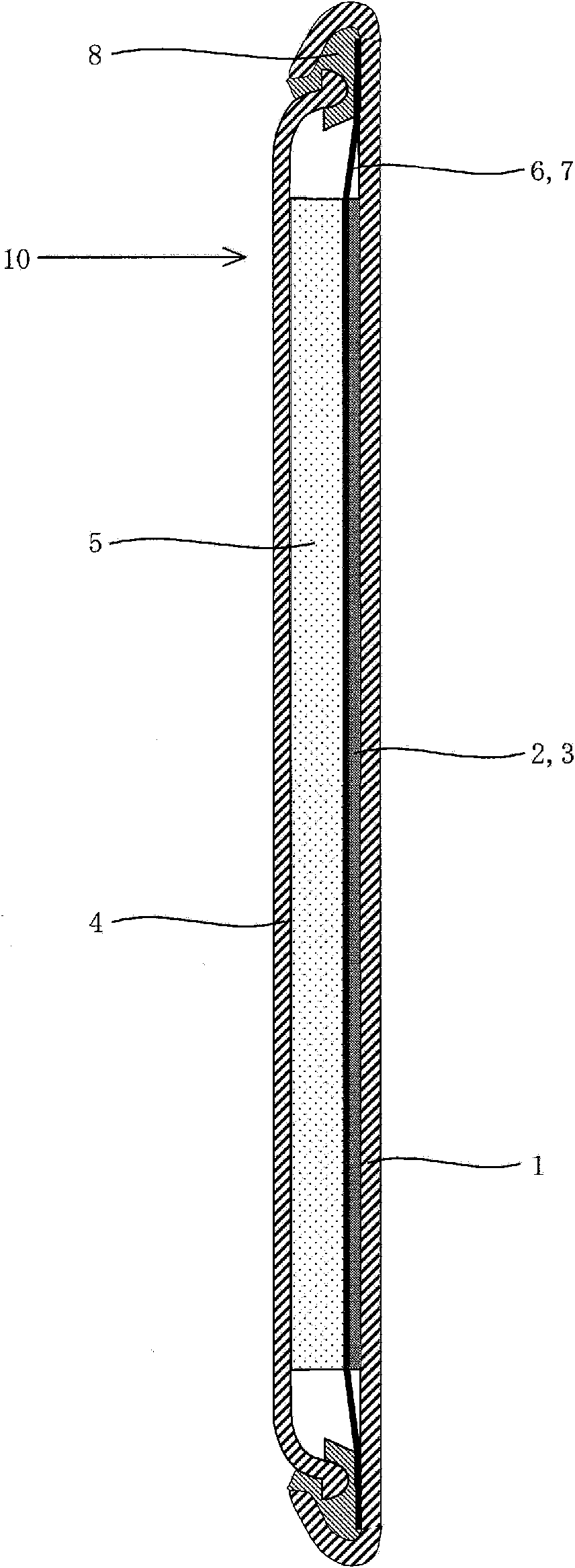
[0146] figure 1is a cross-sectional view showing the structure of the magnesium battery 10 in the embodiment of the present invention.
[0147] like figure 1 As s...
Embodiment 1
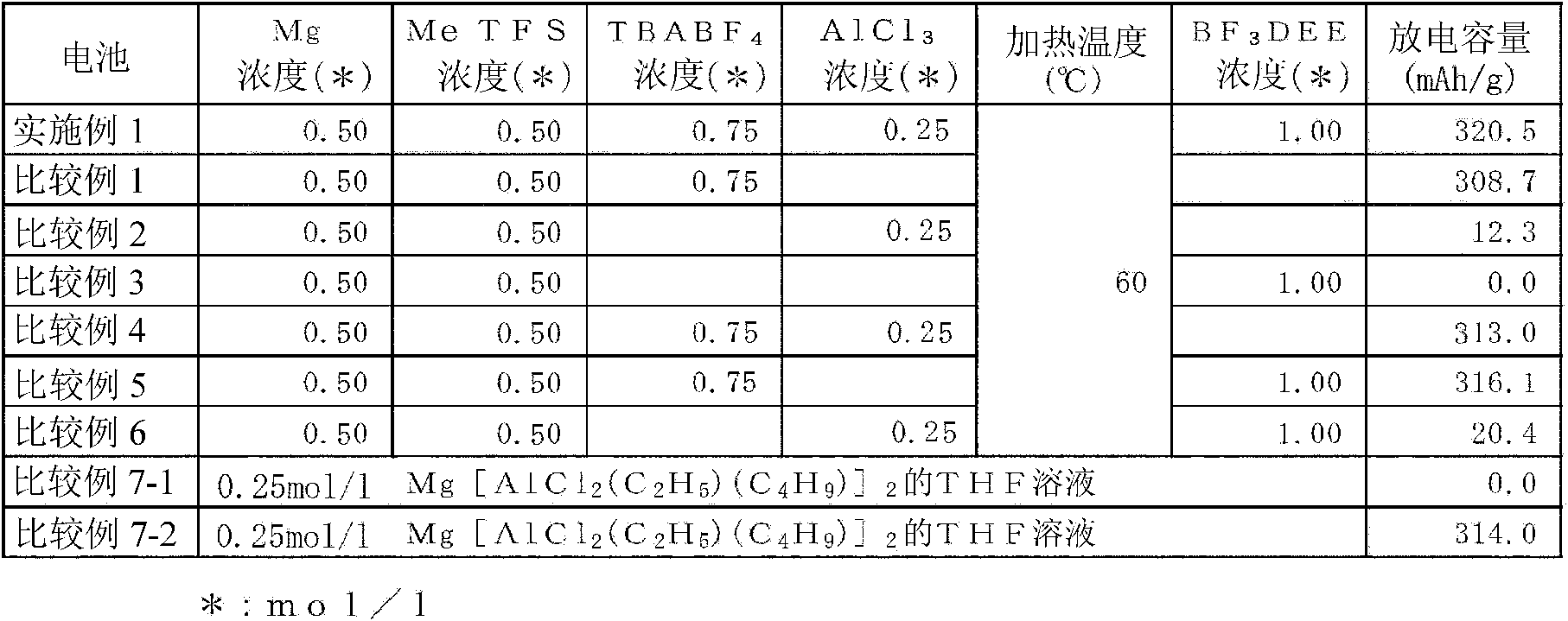
[0172] In Example 1, figure 1 The illustrated coin-type magnesium battery 10 was manufactured by using metallic magnesium as the negative electrode active material 5, by using magnesium oxide as the positive electrode active material, and by using the electrolytic solution according to the present invention, and the performance of the electrolytic solution was examined.
[0173]
[0174] 0.12 g of metallic magnesium was added to 10 ml of 1,2-dimethoxyethane. In addition, 0.55 ml of methyl trifluoromethanesulfonate (MeTFS), 2.47 g of tetrabutylammonium tetrafluoroborate (TBABF 4 ), and 0.33g of aluminum chloride (AlCl 3 ). This corresponds to magnesium metal, methyl trifluoromethanesulfonate, tetrabutylammonium tetrafluoroborate and aluminum chloride at a ratio of 0.50 mol / l to 1,2-dimethoxyethane, respectively, at 0.50 mol / l The ratio of 0.75mol / l, and the ratio of 0.25mol / l was added. Heat treatment was performed at 60° C. for 20 hours while stirring them, so that both ...
Embodiment 2
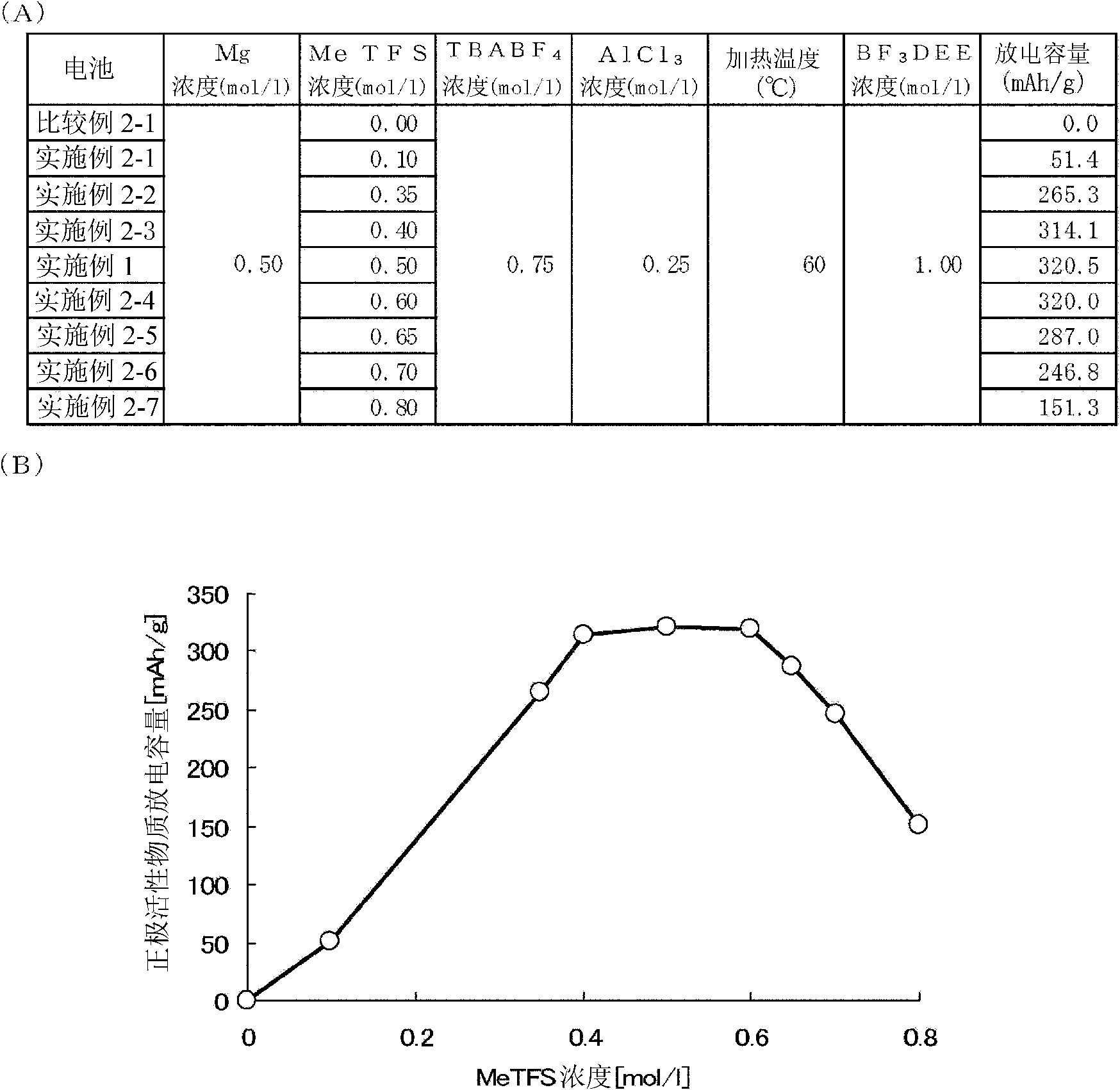
[0198] In Example 2, the concentration of methyl triflate was changed in the range of 0 mol / l to 0.80 mol / l, and thus an electrolytic solution was prepared. Except for this point, a magnesium battery 10 using an electrolytic solution was manufactured similarly to the case of Example 1, and a discharge test was performed on the magnesium battery 10 .
[0199] image 3 It shows the Mg, MeTFS, TBABF used in the synthesis electrolyte solution in Comparative Example 2-1, Example 2-1 to Example 2-7 and Example 1 of the present invention 4 , AlCl 3 , and BF 3 A graph showing the relationship between the concentration of DEE, heating temperature, and the discharge capacity of a magnesium battery using the synthesized electrolyte.
[0200] image 3 (A) in is a numerical table showing the above-mentioned concentration, heating temperature and discharge capacity, and image 3 (B) in image 3 A graph in which the discharge capacity shown in (A) is expressed in the form of a curve. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com