Boron-doped lithium nickel cobaltate anode material
A lithium nickel cobalt oxide and positive electrode material technology, which is applied in the direction of electrical components, battery electrodes, nickel compounds, etc., can solve the problem of the uniformity of material crystallization, the difficulty of precise control of particle shape and particle size distribution, increased energy consumption, exhaust gas emissions, etc. problems, to achieve the effect of improving the first discharge efficiency, improving electrochemical performance, and improving structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Weigh the reaction material LiOH·H respectively according to the ratio of Li:(Ni+Co):B molar ratio of 1.03:0.97:0.03 2 O. Nio 0.8 co 0.2 (OH) 2 and H 3 BO 3 , for thorough mixing of grinding;
[0033] 2) Put the above mixture into a flat-bottomed crucible, heat it in an electrically heated cylindrical high-temperature furnace, use a blower to continuously blow air into the high-temperature furnace, keep the air pressure at 1 standard atmospheric pressure, and heat up with a constant current. When the temperature rises to 500 ℃, keep warm for pretreatment, and the pretreatment time is 5 hours; cool the flat-bottomed crucible to below 100℃ with the furnace, take out the pre-burned raw materials, put them into the mortar and grind again;
[0034] 3) Put the above-mentioned ground raw materials into a rotary muffle furnace, heat up with a constant current, and at the same time feed oxygen, the pressure is 1 standard atmospheric pressure, the temperature rises to 750...
Embodiment 2
[0039] The preparation process of this embodiment is the same as that of Example 1, except that the Ni 0.8 co 0.2 (OH) 2 Change to LiNi 0.8 co 0.2 o 2 .and LiOH·H 2 The amount of O is changed to H 3 BO 3 Half the number of moles used.
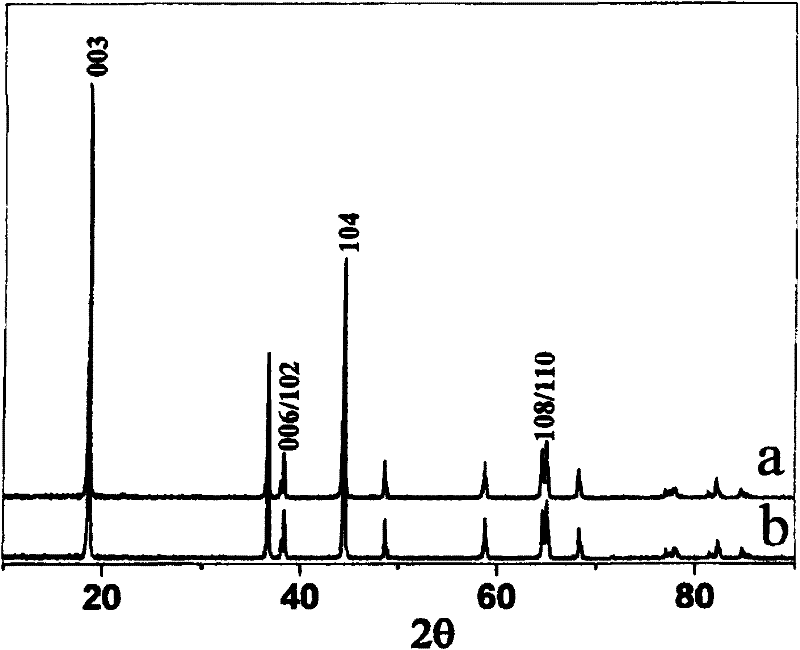
[0040] The resulting product is LiNi 0.776 co 0.194 B 0.03 o 2 , where the X-ray diffraction pattern is as image 3 Shown in b in, take the synthetic product of above-mentioned preparation method to be spherical (see figure 2 ), the average particle size is 8μm, and the tap density is 2.5g / cm3. After making a simulated battery, test its capacity and cycle performance, such as Figure 4 and Figure 5 As shown in the curve b, under the 3.0-4.3V, 0.5C rate charge and discharge system, the discharge specific capacity is 188.8mAh / g, and the capacity retention rate after 50 cycles under the 1C rate charge and discharge system is 93.3%.
Embodiment 3
[0042] The raw materials used in this embodiment are the same as in Example 1, and the operation steps are slightly changed:
[0043] Li in step 1): (Ni+Co): the mol ratio of B is changed into 1.03: 0.99: 0.01;
[0044] The heat preservation temperature in step 2) was changed to 400° C. for 8 hours;
[0045] The heat preservation temperature in step 4) was changed to 800° C. for 6 hours.
[0046] The resulting product is LiNi 0.792 co 0.198 B 0.01 o 2 , use this material to make a simulated battery and test its capacity and cycle performance, such as Figure 6 and Figure 7 As shown in the curve b, under the 3.0-4.3V, 0.5C rate charge and discharge system, the discharge specific capacity is 187.5mAh / g, and the capacity retention rate is 89% after 50 cycles under the 1C rate charge and discharge system.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com