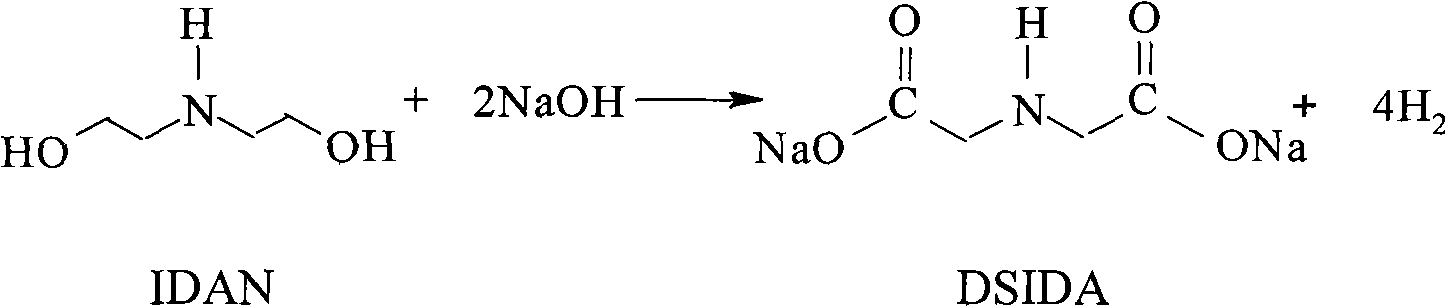Synthesis as well as tail gas treatment technology and device of iminodiacetate
A technology of iminodiacetate and synthesis process, which is applied in the preparation of organic compounds, organic chemistry, and cyanide reaction preparation, etc., which can solve the problems of cumbersome operation, numerous recovery procedures, and low hydrogen recovery rate, and achieve process operation Simple, thorough material reaction, beneficial to the effect of reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
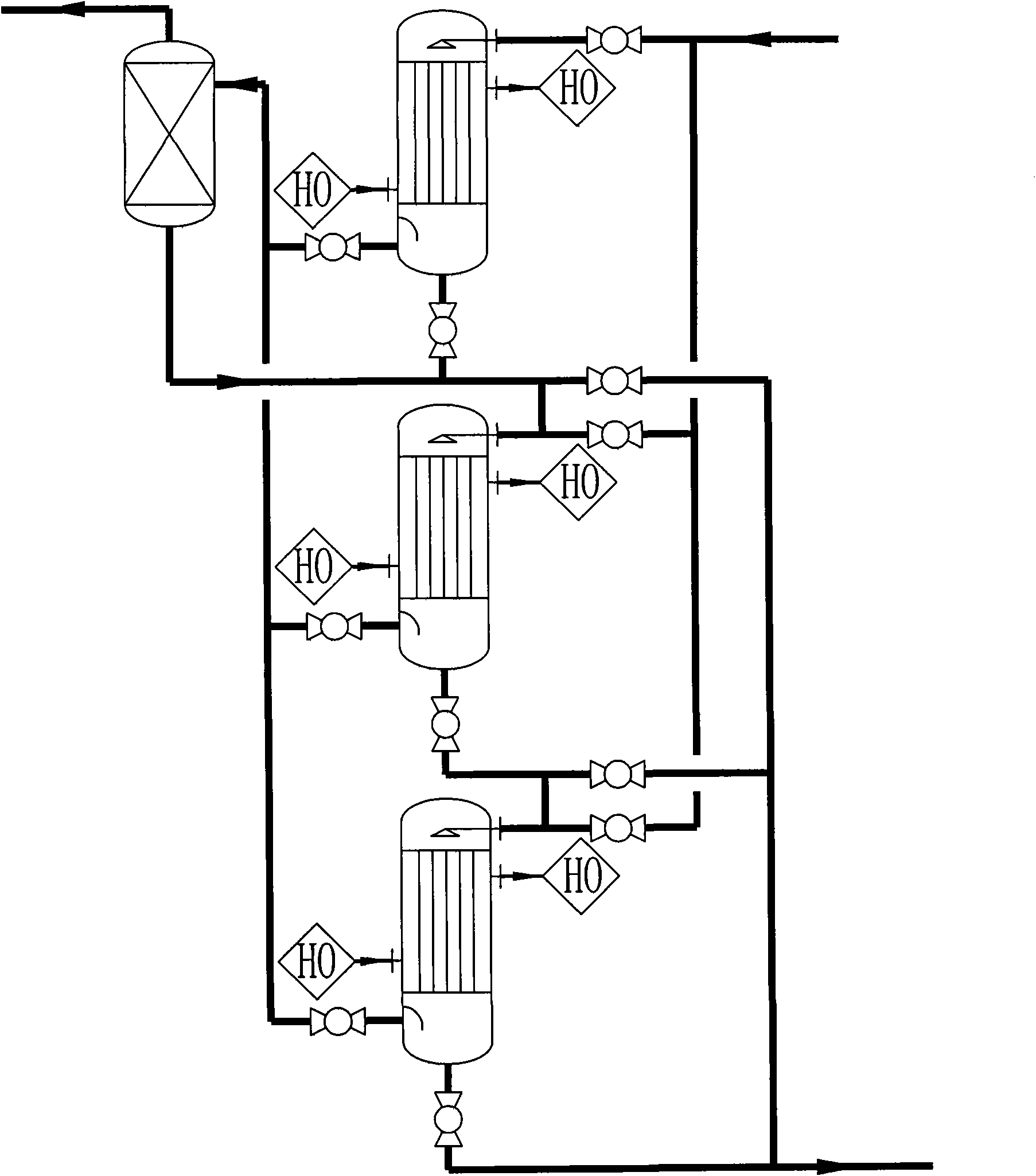
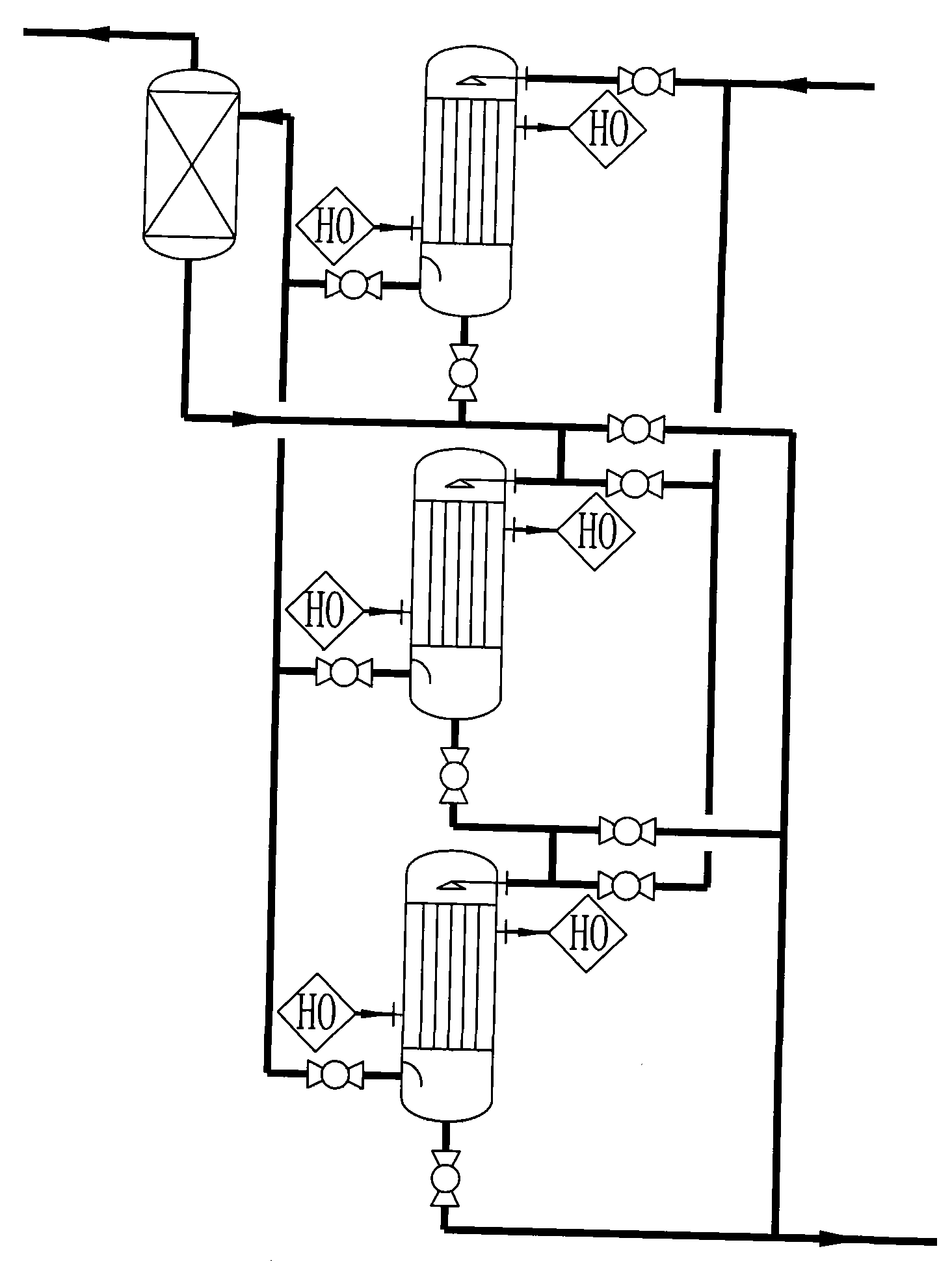
Image
Examples
Embodiment 1
[0035] Diethanolamine, liquid caustic soda, and deionized water are uniformly mixed in proportion to prepare a feed solution with a mass concentration of 20%. Use a metering pump to pump the feed liquid into a multi-stage tubular reactor filled with solid catalysts at a flow rate of 150Kg / h. The feed liquid is heated by a three-stage heater before entering the reactor. The material temperature is controlled at 175°C, the temperature in the reactor is controlled between 170 and 175°C, and the pressure in the reactor is maintained at 1.80Mpa. The liquid output from the gas-liquid separator enters the DSIDA metering tank, and the DSIDA yield reaches about 98.2%. The tail gas enters the water washing device through the pipeline after the regulating valve. The mixed gas obtained by water washing with a composition of 96.0% hydrogen and 3.9% water enters a drying device for drying treatment. The desiccant is molecular sieve, and 99.9% hydrogen is obtained, and the hydrogen recovery ...
Embodiment 2
[0037] Diethanolamine, liquid caustic soda, and deionized water are uniformly mixed in proportion to prepare a feed solution with a mass concentration of 20%. Use a metering pump to pump the feed liquid into a multi-stage tubular reactor filled with solid catalysts at a flow rate of 150Kg / h. The feed liquid is heated by a three-stage heater before entering the reactor. The material temperature is controlled at 155°C, the temperature in the reactor is controlled between 150 and 160°C, and the pressure in the reactor is maintained at 1.80Mpa. The liquid output from the gas-liquid separator enters the DSIDA metering tank, and the DSIDA yield reaches about 97.0%. The tail gas enters the water washing device through the pipeline after the regulating valve. The mixed gas obtained by water washing with a composition of 95.5% hydrogen and 4.4% water enters a drying device for drying treatment. The desiccant is molecular sieve, and 99.85% hydrogen is obtained, and the hydrogen recovery...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com