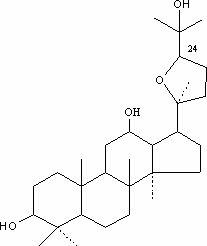
Preparation method of 24(R)-ocotillol DQ and pharmacal application thereof
A technology of ginsenosides and aglycones, which is applied in the field of medicine and can solve problems such as no curative effect and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Take 1.0 kg of total ginsenosides, add water to dissolve, add to the treated macroporous adsorption resin column AB-8, elute with 35% ethanol until no saponins are reacted, and then elute with 60% ethanol;
[0023] (2) After the eluent is evaporated to dryness, add sodium hydroxide for hydrolysis, adjust the pH of the hydrolyzate to neutral, add it to the macroporous adsorption resin column AB-8, and elute with 60-90% ethanol after desalination;
[0024] (3) After the eluent was evaporated to dryness, H 2 o 2 Oxidation, adjust the pH of the oxide to neutral, and precipitate with water; the precipitation is subjected to silica gel column chromatography, eluted with petroleum ether-ethyl acetate 1:1, and the eluate containing 24(R)-pseudoginsenoside DQ is collected, The solvent was recovered to dryness and recrystallized with methanol to obtain the product.
Embodiment 2
[0026] (1) Take 1.0kg of total saponins from ginseng stems and leaves, add water to dissolve, add to the treated macroporous adsorption resin D 101 On the column, elute with 45% ethanol until there is no saponin reaction, and then elute with 75% ethanol;
[0027] (2) After the eluent is evaporated to dryness, add sodium hydroxide for hydrolysis, adjust the pH of the hydrolyzate to neutral, add it to the macroporous adsorption resin column AB-8, and elute with 60-90% ethanol after desalination;
[0028] (3) After the eluent is evaporated to dryness, it is oxidized with m-chloroperoxybenzoic acid, the pH of the oxide is adjusted to neutral, and it is precipitated with water; the precipitate is subjected to silica gel column chromatography, and is eluted with petroleum ether-ethyl acetate 3:1 , collect the eluate containing 24(R)-pseudoginsenoside DQ, recover the solvent to dryness, and recrystallize from ethanol to obtain the product.
Embodiment 3
[0030] (1) Take 1.0kg of total saponins from American ginseng stems and leaves, add water to dissolve, add to the treated macroporous adsorption resin D 4020 On the column, elute with 55% ethanol until there is no saponin reaction, and then elute with 90% ethanol;
[0031] (2) After the eluent is evaporated to dryness, add sodium hydroxide for hydrolysis, adjust the pH of the hydrolyzate to neutral, add it to the macroporous adsorption resin column AB-8, and elute with 90% ethanol after desalination;
[0032] (3) After the eluent was evaporated to dryness, potassium permanganate was oxidized, and the pH of the oxide was adjusted to neutral, and precipitated with water; the precipitate was subjected to silica gel column chromatography, eluted with petroleum ether-ethyl acetate 5:1, and the collected The eluate of 24(R)-pseudoginsenoside DQ, the solvent was recovered to dryness, and the product was obtained by recrystallization from ethyl acetate.
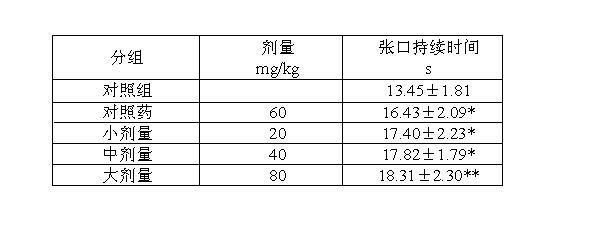
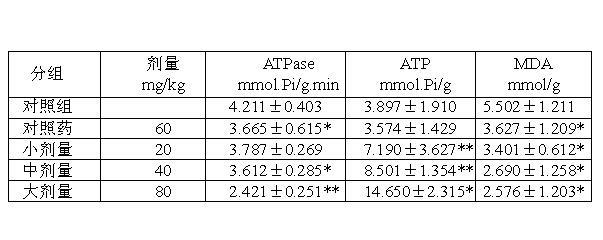
[0033] Below in conjunction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com