Azithromycin sustained-release microsphere and dry suspension, preparation method and application thereof
A technology of azithromycin and slow-release microspheres, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, respiratory diseases, etc., and can solve the problems of unsuitable simple, environmentally friendly and non-toxic extrusion-spheronization methods, etc., to achieve Good sustained release effect, reduced dosage, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
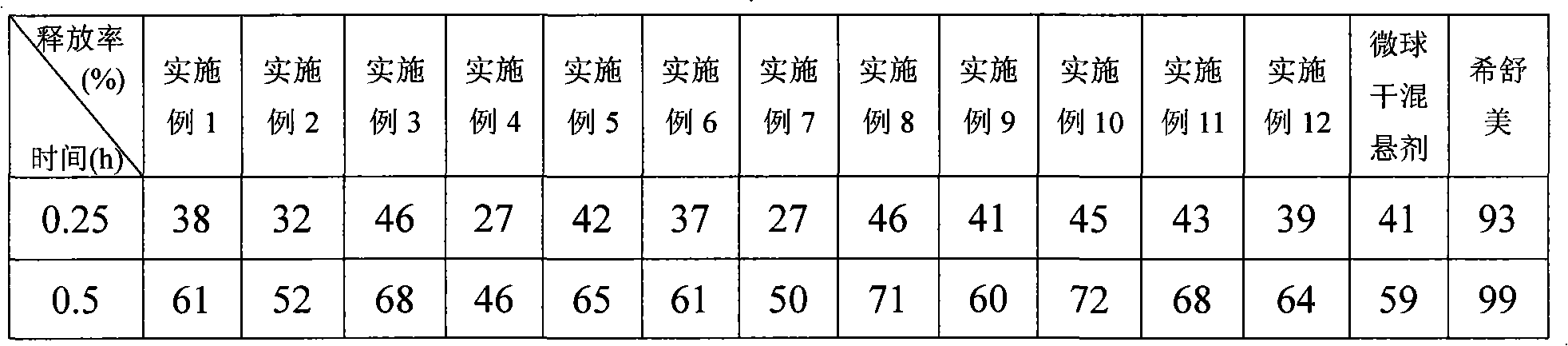
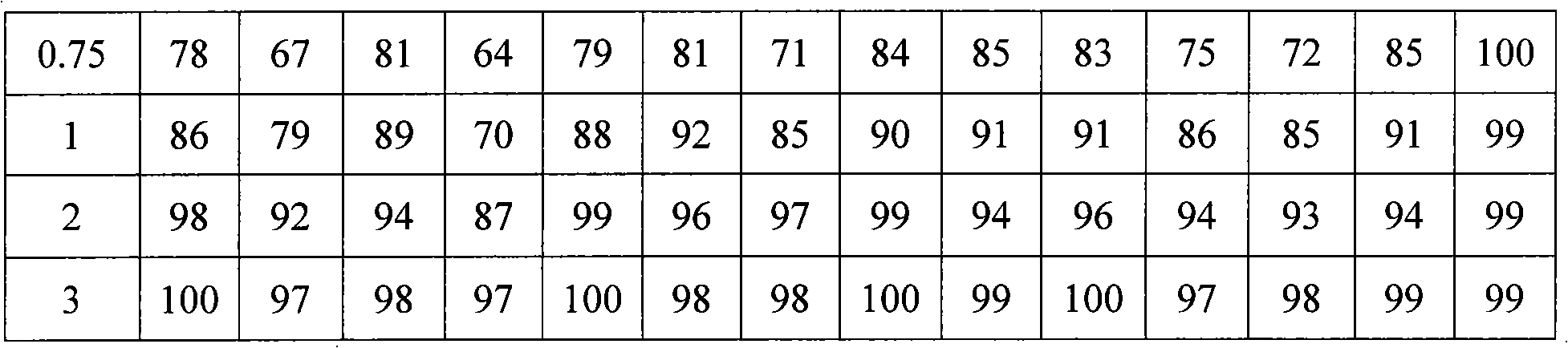
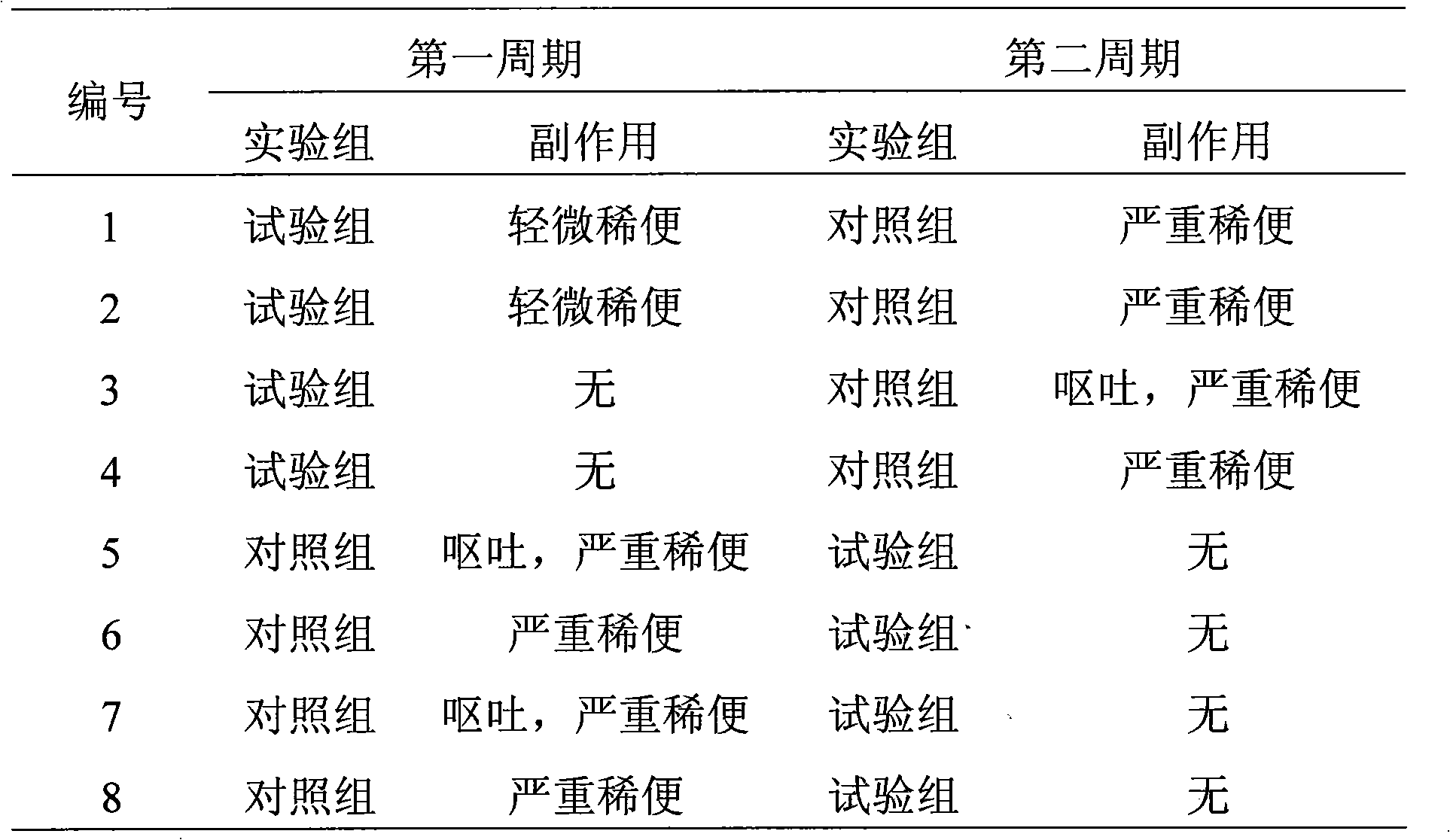
Examples
Embodiment 1
[0047] 1. The formulation of azithromycin sustained-release microspheres:
[0048] Azithromycin 52%, stearyl alcohol 16%, microcrystalline cellulose 8%, ethyl cellulose 9%, and ethyl cellulose aqueous dispersion 15% (content of solid matter).
[0049] 2. Preparation process of azithromycin sustained-release microspheres:
[0050] Adopt the known method of pharmaceutical industry, mix azithromycin with stearyl alcohol, microcrystalline cellulose and ethyl cellulose according to the above formula, add binder ethyl cellulose aqueous dispersion (Surelease ) to prepare a soft material, extrude it with a sieve plate with an aperture of 0.2mm, and carry out spheronization with a rotating speed of 900rpm. The obtained microspheres were dried in an oven at 40°C for 2 hours to remove moisture, and then heat-treated at 70°C for 2 hours. After cooling to room temperature, the particles were sized to collect microspheres with particle diameters between 60 and 100 mesh, and the yield was...
Embodiment 2
[0052] 1. The formulation of azithromycin sustained-release microspheres:
[0053] Azithromycin 35%, glyceryl monostearate 25%, microcrystalline cellulose 7%, ethyl cellulose 22.9%, and ethyl cellulose aqueous dispersion 0.1% (content of solid matter).
[0054] 2. Preparation process of azithromycin sustained-release microspheres:
[0055] Using methods known in the pharmaceutical industry, azithromycin is mixed with glyceryl monostearate, microcrystalline cellulose and ethyl cellulose according to the above formula, and the binder ethyl cellulose aqueous dispersion (Aquacoat ) to prepare a soft material, extrude it with a sieve plate with a pore size of 0.2 mm, and carry out spheronization with a rotating speed of 1200 rpm. The obtained microspheres were dried in an oven at 40°C for 1 hour to remove moisture, and then heat-treated at 70°C for 2 hours. After cooling to room temperature, the particles were sized to collect microspheres with a particle size between 60 and 100...
Embodiment 3
[0057] 1. The formulation of azithromycin sustained-release microspheres:
[0058] Azithromycin 70%, Glyceryl Behenate 18%, Ethylcellulose 5%, and Ethylcellulose aqueous dispersion 7% (solid matter content).
[0059] 2. Preparation process of azithromycin sustained-release microspheres:
[0060] Adopt the known method of pharmaceutical industry, mix azithromycin and glyceryl behenate according to the above-mentioned formula, add binder ethyl cellulose aqueous dispersion (Yishida ) to prepare a soft material, extrude it with a sieve plate with an aperture of 0.25mm, and carry out spheronization with a rotating speed of 500rpm. The obtained microspheres were heat-treated at 75°C for 3 hours. After cooling to room temperature, the particles were sized to collect microspheres with a particle size between 60 and 100 mesh. The yield is 91%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com