Porous cubic phase scandium oxide powder and preparation method thereof
A technology of scandium oxide and cubic phase, applied in chemical instruments and methods, inorganic chemistry, rare earth metal compounds, etc., can solve problems such as poor dispersion and uneven size distribution, and achieve good performance and narrow particle size distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
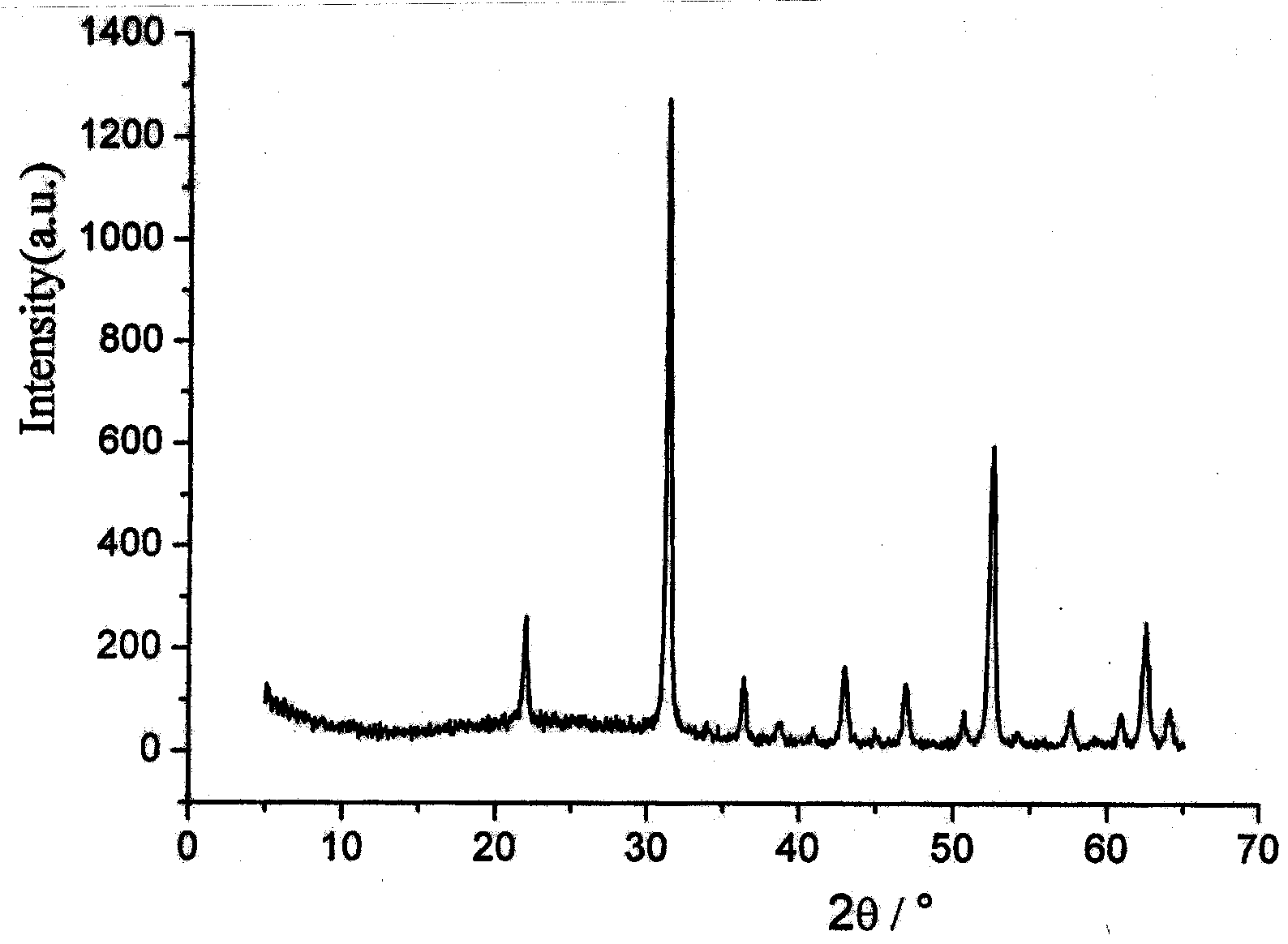
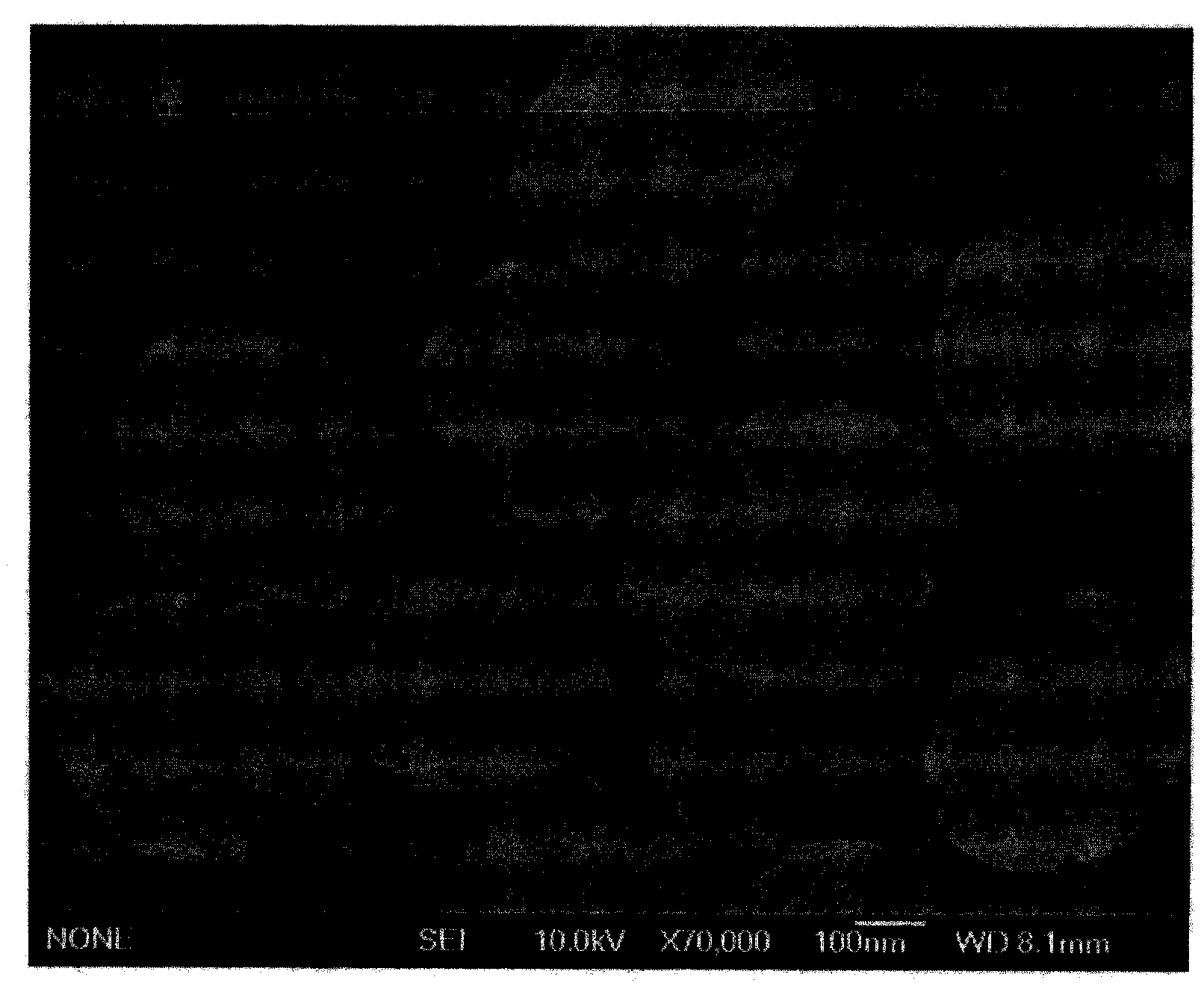
[0021] Add 0.138 g of scandium oxide and 1 ml of concentrated nitric acid to 10 ml of distilled water, and heat and stir to dissolve. After the dissolution is complete, the solution is evaporated to dryness to remove excess concentrated nitric acid to obtain dry white scandium nitrate. Then add 20 ml of distilled water to it, stir and dissolve at room temperature, and add 0.410 g of sodium acetate trihydrate, 0.500 g of polyethylene glycol 1000 and 1.000 g of hexamethylenetetramine to the aqueous solution of scandium nitrate while stirring. After the complete dissolution, it was poured into a 30ml reaction kettle and kept at a constant temperature of 100°C for 24h. The obtained white precipitate was collected by filtration and dried at 65°C. Finally, the obtained white precipitate was calcined at 800°C for 4 hours to obtain a white powder. The obtained white powder is confirmed to be cubic scandium oxide by X-ray diffraction analysis, such as figure 1 ; Scanning electron mic...
example 2
[0023] Add 0.138 g of scandium oxide and 1 ml of concentrated nitric acid to 10 ml of distilled water, and heat and stir to dissolve. After the dissolution is complete, the solution is evaporated to dryness to remove excess concentrated nitric acid to obtain dry white scandium nitrate. Then, 20 ml of distilled water was added thereto, and the mixture was stirred and dissolved at room temperature. Then, 0.410 g of sodium acetate trihydrate, 0.500 g of polyethylene glycol 1000 and 1.000 g of hexamethylenetetramine were added to the aqueous solution of scandium nitrate while stirring. After the complete dissolution, it was poured into a 30ml reaction kettle and kept at a constant temperature of 100°C for 4h. The obtained white precipitate was collected by filtration and dried at 65°C. Finally, the obtained white precipitate was calcined at 800°C for 4 hours to obtain a white powder. The obtained white powder was confirmed to be cubic scandium oxide by X-ray diffraction analysis....
example 3
[0025] Add 0.138 g of scandium oxide and 1 ml of concentrated nitric acid to 10 ml of distilled water, and heat and stir to dissolve. After the dissolution is complete, the solution is evaporated to dryness to remove excess concentrated nitric acid to obtain dry white scandium nitrate. Then, 20 ml of distilled water was added thereto, and the mixture was stirred and dissolved at room temperature. Then, 0.410 g of sodium acetate trihydrate, 0.500 g of polyethylene glycol 1000 and 1.000 g of hexamethylenetetramine were added to the aqueous solution of scandium nitrate while stirring. After the complete dissolution, it was poured into a 30ml reaction kettle and kept at a constant temperature of 100°C for 48h. The obtained white precipitate was collected by filtration and dried at 65°C. Finally, the obtained white precipitate was calcined at 800°C for 4 hours to obtain a white powder. The obtained white powder was confirmed to be cubic scandium oxide by X-ray diffraction analysis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com