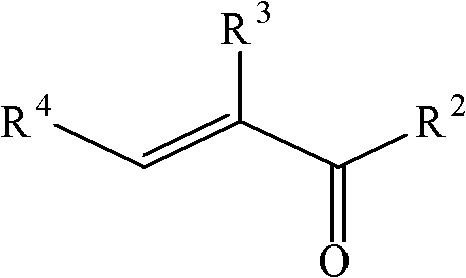
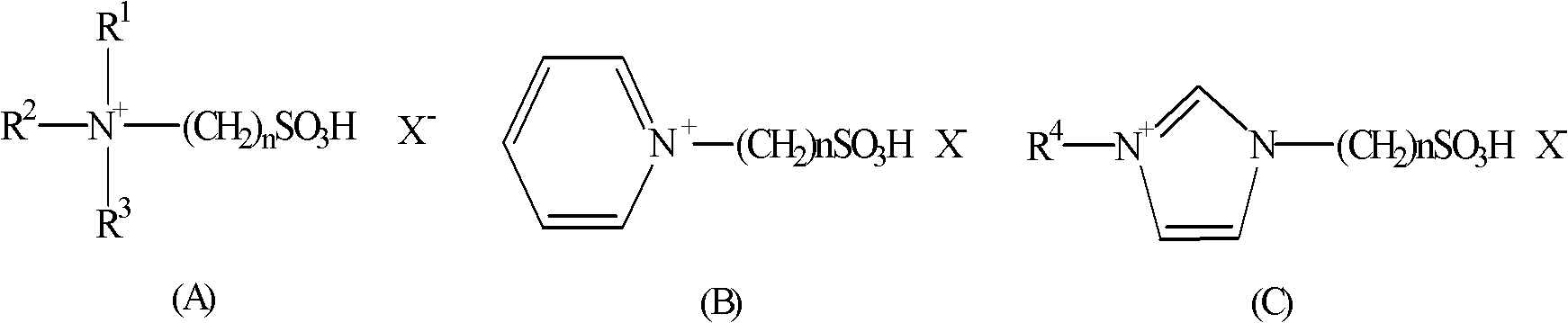Method for preparing quinoline derivative
A technology of derivatives and quinoline, which is applied in the field of preparation of quinoline derivatives, can solve the problems of difficult post-processing of glycerin, inconvenient follow-up processing, and low yield of quinoline, etc., and achieve easy product, mild reaction conditions, and reaction selectivity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of 2-methylquinoline
[0028] In a 0.5 liter reaction flask, add aniline (93 grams, 1 mole), 1-methyl-3-sulfonic acid propylimidazolium bisulfate (100 grams), sodium iodide (5 grams), at 100 ~ 105 ° C Next, crotonaldehyde (77 g, 1.1 mol) was added dropwise, and the addition was completed in 1.5 hours, and the reaction was continued for 1 hour at this temperature. After cooling, water (300 ml) was added to the reaction solution, neutralized with 30% sodium hydroxide to pH=9-10, stirred for 30 minutes, and extracted with toluene.
[0029] The filtrate was adjusted to acidity with sulfuric acid, concentrated, and the ionic liquid was recovered.
[0030] The toluene solution was concentrated and distilled under reduced pressure to collect fractions at 118-120° C. / 10 mmHg to obtain 133 g of 2-methylquinoline (93% yield).
[0031] The product has a purity of 99.5% as determined by gas chromatography.
Embodiment 2
[0033] Preparation of 3-methylquinoline
[0034] In a 0.5-liter reaction flask, add aniline (93 grams, 1 mole), (3-sulfonic acid propyl) triethylammonium bisulfate (100 grams), potassium iodide (3 grams), drop at 95 ~ 100 ° C α-Methacrolein (77 g, 1.1 mol) was added over 1 hour and the reaction was continued at this temperature for 1 hour. After cooling, water (300 ml) was added to the reaction solution, neutralized with 30% sodium hydroxide to pH=9-10, stirred for 30 minutes, and extracted with toluene. The filtrate was adjusted to acidity with sulfuric acid, concentrated, and the ionic liquid was recovered.
[0035] The toluene solution was concentrated and distilled under reduced pressure to collect fractions at 120-123° C. / 10 mmHg to obtain 126 grams of 3-methylquinoline (88% yield).
[0036] The purity of the product as determined by gas chromatography is 99.4%.
Embodiment 3
[0038] Preparation of 4-methylquinoline
[0039] In a 0.5-liter reaction flask, add aniline (93 grams, 1 mole), 1-methyl-3-sulfonic acid propylimidazolium bisulfate (100 grams), iodine (3 grams), and drop at 90 to 95 ° C Methyl ketene (91 g, 1.3 moles) was added over 1.5 hours and the reaction was continued at this temperature for 2 hours. After cooling, water (300 ml) was added to the reaction solution, neutralized with 30% sodium hydroxide to pH=9-10, stirred for 30 minutes, and extracted with toluene. The filtrate was adjusted to acidity with sulfuric acid, concentrated, and the ionic liquid was recovered.
[0040] The toluene solution was concentrated and distilled under reduced pressure to collect fractions at 130-132° C. / 5 mmHg to obtain 131.5 g of 4-methylquinoline (92% yield).
[0041] The purity of the product as determined by gas chromatography is 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com