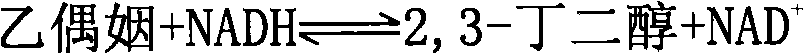(2R, 3R)-2,3-butanediol dehydrogenase and coding gene and application thereof
A technology of butanediol and dehydrogenase, applied in the -2 field, can solve the problems of unsuitability for industrial production of butanediol, no industrial application value, low output and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, (2R, 3R)-2, the construction of the acquisition of 3-butanediol dehydrogenase gene and its prokaryotic expression vector
[0052] The (2R,3R)-2,3-butanediol dehydrogenase gene from Paenibacillus polymyxa ATCC12321 was amplified from the Paenibacillus polymyxa ATCC12321 genome by PCR after whole genome sequencing and annotation analysis . Among them, the Paenibacillus polymyxa ATCC12321 strain was purchased from the American Biological Resource Collection (http: / / www.atcc.org / ), and the public can directly purchase it freely from the American Biological Resource Collection.
[0053] The cloning process of the gene and the construction process of its prokaryotic expression vector comprise the following steps:
[0054] 1. Cloning of (2R,3R)-2,3-butanediol dehydrogenase gene
[0055] After whole genome sequencing and annotation of genome information, a gene that may encode butanediol dehydrogenase was obtained, and the following primers were designed accordi...
Embodiment 2
[0058] Example 2, (2R, 3R)-2,3-butanediol dehydrogenase expression and purification in prokaryotic system
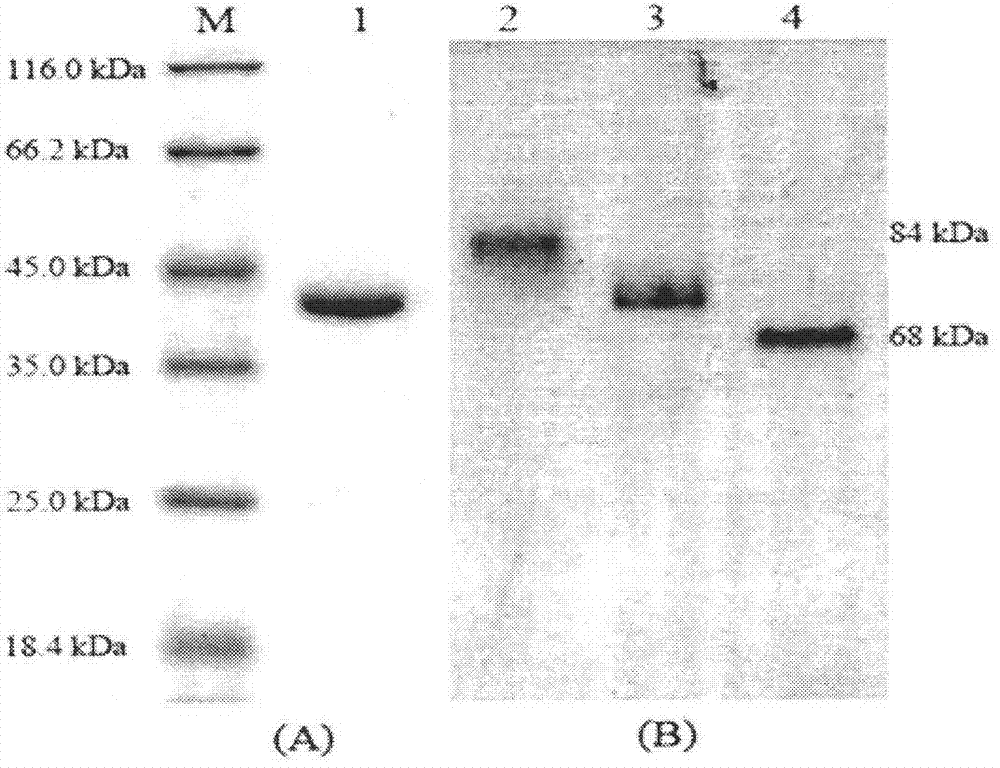
[0059] The prokaryotic expression vector pET22b-2R3R containing the (2R,3R)-2,3-butanediol dehydrogenase gene obtained in Example 1 was transformed into Escherichia coli E.coli BL21(DE3)pLysS, and the positive single clone was selected at 37°C Shake the bacteria until the OD600 is 0.6, add 1mM IPTG to induce, collect the bacteria by centrifugation after 5 hours, resuspend in ultrasonic buffer (containing 20mM phosphate buffer, 300mM NaCl, 10mM imidazole, pH 7.4) after repeated freezing and thawing , sonicated the cells, collected the supernatants and precipitates, and carried out SDS-PAGE electrophoresis analysis. The results showed that the prokaryotic expression vector carrying the target gene was expressed in large quantities in E. coli BL21(DE3)pLysS, and the expressed recombinant protein was single The molecular weight of the subunit is 38kDa, which is consistent wi...
Embodiment 3
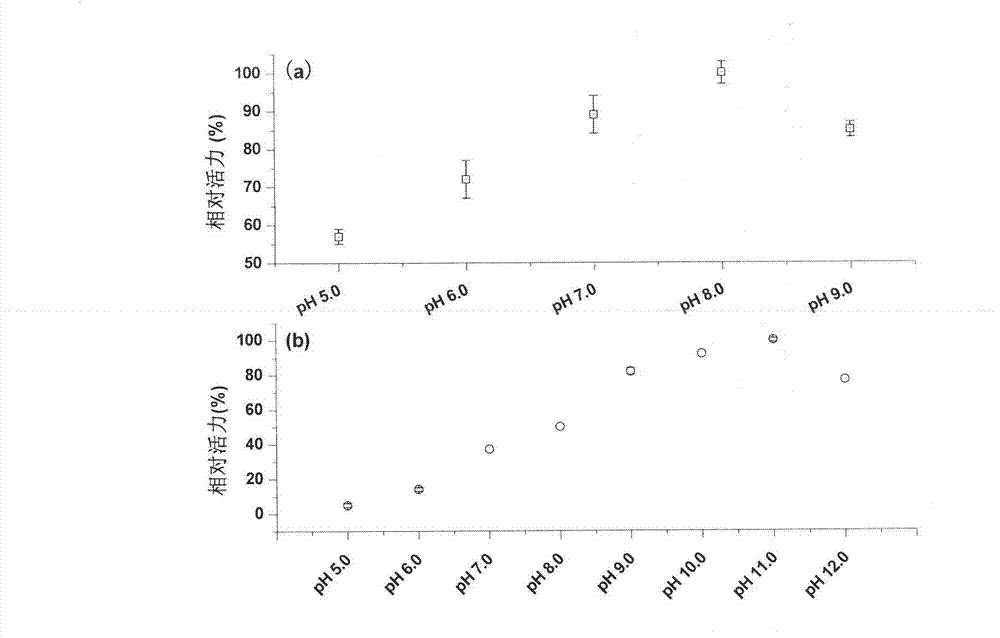
[0061] Embodiment 3, enzymatic characteristic identification
[0062] Enzyme characterization was performed using the following reactions:
[0063]
[0064] This reaction is a reversible reaction, and the change of NADH is used as the basis for the determination of enzyme activity (the obtained recombinant protein can only use NAD+ / NADH as a coenzyme, and cannot use NADP+ / NADPH as a coenzyme).
[0065] Using NAD+ as a coenzyme, catalyze (2R,3R)-2,3-butanediol to (R)-acetoin (at 70°C, pH 11.0, with 100mM (2R,3R)-2,3- Butanediol is a substrate, 4mM NAD+ is a coenzyme), the result only generates (R)-acetoin; catalyzes meso-2,3-butanediol to generate (S)-acetoin (at 70 Under the conditions of ℃ and pH 11.0, using 100mM meso-2,3-butanediol as the substrate and 4mM NAD+ as the coenzyme), only (S)-acetoin was produced.
[0066] Using NADH as a coenzyme, catalyze (R)-acetoin to generate (2R,3R)-2,3-butanediol (at 70°C, pH 8.0, with 10mM (R)-acetoin as substrate , 0.2mM NADH is a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com