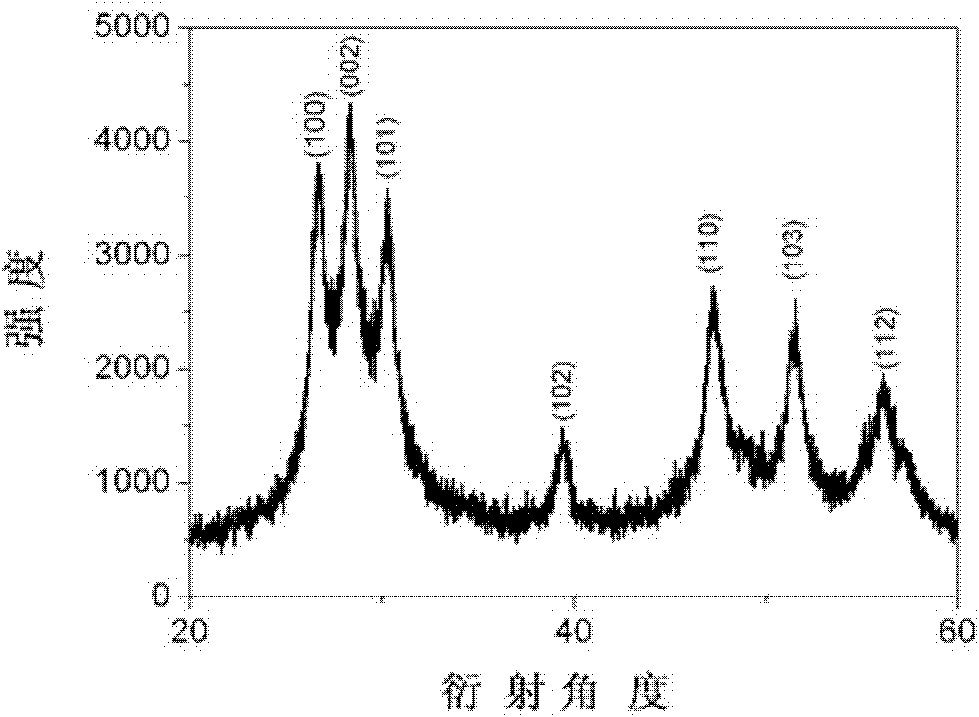
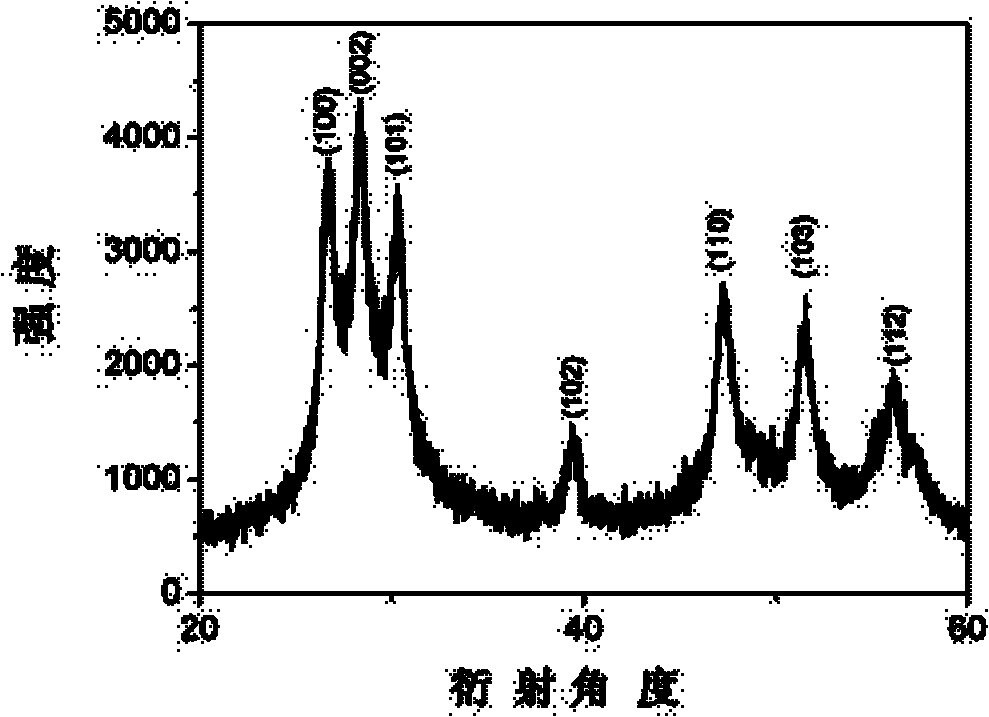
Method for preparing doping type hexagonal system nano ZnS at low temperature
A technology of hexagonal crystal system and doping type, which is applied in the field of preparation of hexagonal crystal β-ZnS, can solve the problems of high temperature required for the reaction, incomplete hexagonal ZnS crystal form, etc., and achieve regular product shape and size Uniform, easy-to-handle results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] First, make 0.5g of zinc acetate into a 3mL aqueous solution and add it to a 50mL three-neck round-bottomed flask, stir continuously, then add the copper acetate used for doping into the prepared zinc acetate aqueous solution [n(Zn 2+ ): n (Cu 2+ ) = 50:1], followed by adding 21mL of DMF, and then adding 0.6mL of surfactant thioglycolic acid [n (Zn 2+ ): n (thioglycolic acid) = 1:3.8], then adjust the pH value of the mixed solution with ammonia water, adjust the pH to about 8.5, and finally add 3mL thiourea (0.1734g) DMF solution [n (thiourea): n (Zn 2+ ) = 1:1]. The above-mentioned process of adding the reaction raw materials has been kept in a stirring state at room temperature. However, the course of the reaction was refluxed at 90°C for 14 hours after all material had been added. After the reaction is completed, add acetone twice the volume of the solution to obtain a precipitate, centrifuge to separate the obtained precipitate, and wash the precipitate four tim...
Embodiment 2
[0027] First, make 3mL aqueous solution of 0.5g zinc acetate into a 50mL three-neck round-bottomed flask, stir constantly, then add manganese acetate used for doping into the prepared zinc acetate aqueous solution [n(Zn 2+ ):n(Mn 2+ ) = 100:1], followed by adding 21mL of DMF, and then adding 0.6mL of surfactant thioglycolic acid [n (Zn 2+ ): n (thioglycolic acid) = 1:3.8], then adjust the pH value of the mixed solution with ammonia water, adjust the pH to about 8.5, and finally add 3mL thiourea (0.1734g) DMF solution [n (thiourea): n (Zn 2+ ) = 1:1]. The above-mentioned process of adding the reaction raw materials has been kept in a stirring state at room temperature. However, the course of the reaction was refluxed at 100°C for 5 hours after all material had been added. After the reaction is completed, add acetone twice the volume of the solution to obtain a precipitate, centrifuge to separate the obtained precipitate, and wash the precipitate four times with absolute eth...
Embodiment 3
[0029] First, make 3mL aqueous solution of 0.5g zinc acetate into a 50mL three-necked round-bottomed flask, stir continuously, then add terbium chloride used for doping into the prepared aqueous zinc acetate solution [n(Zn 2+ ): n (Tb 3+) = 150:1], followed by adding 21mL of DMF, and then adding 0.6mL of surfactant thioglycolic acid [n (Zn 2+ ): n (thioglycolic acid) = 1:3.8], then adjust the pH value of the mixed solution with ammonia water, adjust the pH to about 8.5, and finally add 3mL thiourea (0.1734g) DMF solution [n (thiourea): n (Zn 2+ ) = 1:1]. The above-mentioned process of adding the reaction raw materials has been kept in a stirring state at room temperature. However, the course of the reaction was refluxed at 95°C for 14 hours after all material had been added. After the reaction is completed, add acetone twice the volume of the solution to obtain a precipitate, centrifuge to separate the obtained precipitate, and wash the precipitate four times with absolute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com