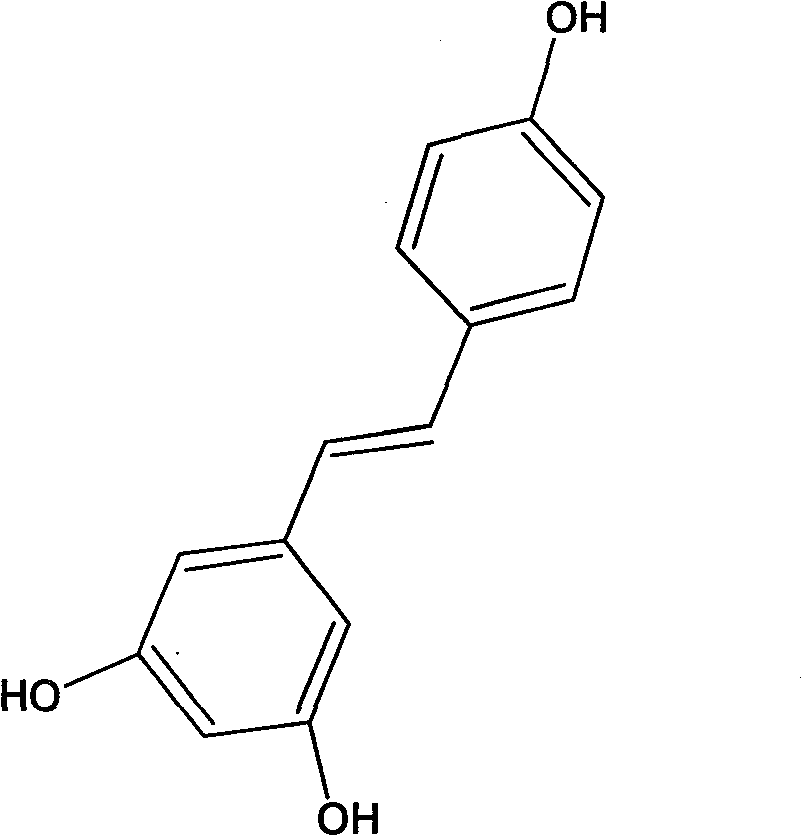
Method for synthesizing trans-resveratrol by combining hydroxyl groups and protective groups
A hydroxyl protecting group and resveratrol technology, which is applied in chemical instruments and methods, preparation of organic compounds, bulk chemical production, etc., can solve the disadvantages of resveratrol, reduce reaction temperature and reduce product cost , The effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 3.5 grams of p-hydroxybenzaldehyde was dissolved in 100 milliliters of acetone, and 7.4 grams of potassium carbonate and 6.4 grams of benzyl bromide were added. After mixing, react at 50° C. for 24 hours under the protection of argon. After cooling to room temperature, it was filtered and concentrated in vacuo. The residue was dissolved in ethyl acetate of the same volume as the reaction system, washed with an equal volume of 1M hydrochloric acid, then washed with an equal volume of saturated brine, dried over anhydrous sodium sulfate or anhydrous magnesium sulfate, filtered, and concentrated to dryness to obtain the intermediate 7.9 grams of p-benzyloxybenzaldehyde (containing impurities such as benzyl bromide).
[0017] 7.9 g of p-benzyloxybenzaldehyde containing benzyl bromide and other impurities, dissolved in 120 ml of methanol, slowly added 1.3 g of NaBH at 0-5 °C 4 , After 1.5 hours of reaction, the reaction was terminated with acetic acid. Methanol was recove...
Embodiment 2
[0021] 30.2 grams of p-hydroxybenzaldehyde was dissolved in 800 milliliters of acetone, and 64 grams of potassium carbonate and 46 grams of benzyl bromide were added. After mixing, react at 50° C. for 12 hours under the protection of argon. After cooling to room temperature, it was filtered and concentrated in vacuo. The residue was dissolved in ethyl acetate of the same volume as the reaction system, washed with an equal volume of 1M hydrochloric acid, then washed with an equal volume of saturated brine, dried over anhydrous sodium sulfate or anhydrous magnesium sulfate, filtered, and concentrated to dryness to obtain the intermediate 66.2 grams of p-benzyloxybenzaldehyde (containing impurities such as benzyl bromide).
[0022] 66.2 grams of p-benzyloxybenzaldehyde containing benzyl bromide and other impurities, dissolved in 800 ml of methanol, slowly added 10 grams of NaBH at 0-5 °C 4 , After 2 hours of reaction, the reaction was terminated with acetic acid. Methanol was ...
Embodiment 3
[0026] 3.4 g of 3,5-dimethoxybenzaldehyde, dissolved in 40 ml of methanol, slowly added 0.5 g of NaBH at 0-5°C 4 , After 1.5 hours of reaction, the reaction was terminated with acetic acid. Methanol was recovered, 8 ml of ice water was added, cooled, and 3.2 g of intermediate 3,5-dimethoxybenzyl alcohol was obtained by filtration.
[0027] 3.2 g of 3,5-dimethoxybenzyl alcohol was stirred and dissolved in 40 ml of dichloromethane, protected by argon. The temperature was controlled below 0°C, and 1 ml of phosphorus tribromide was slowly added dropwise (5 ml of dichloromethane was dissolved and then added dropwise). After dropping, keep at 0°C for 1 hour, then cool to room temperature, pour into a container filled with 60 grams of ice, and separate layers after the ice completely melts. The organic layer (lower layer) was separated, and the aqueous layer was extracted twice with dichloromethane. The organic phases were combined, washed with water until neutral, dried over anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com