Prokaryotic expression vector contributing to purification of foreign protein and construction method thereof
A prokaryotic expression and construction method technology, applied in the field of genetic engineering, can solve problems such as high price affinity chromatography column
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
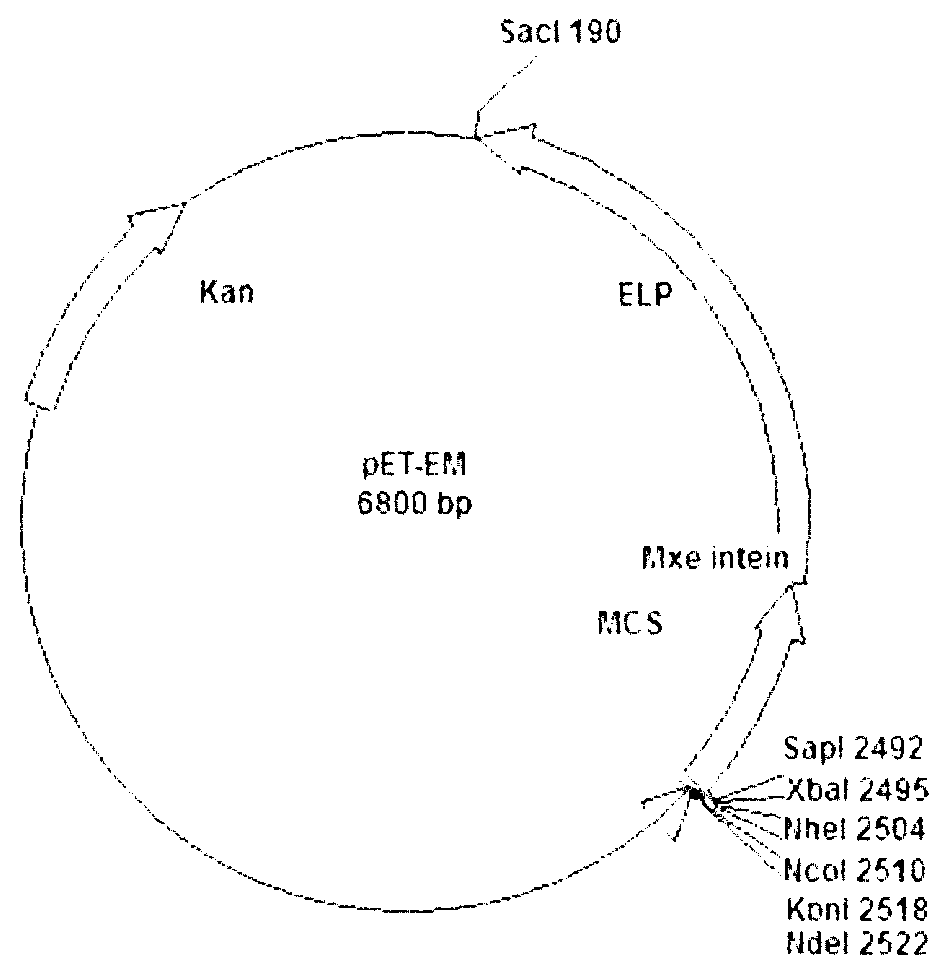
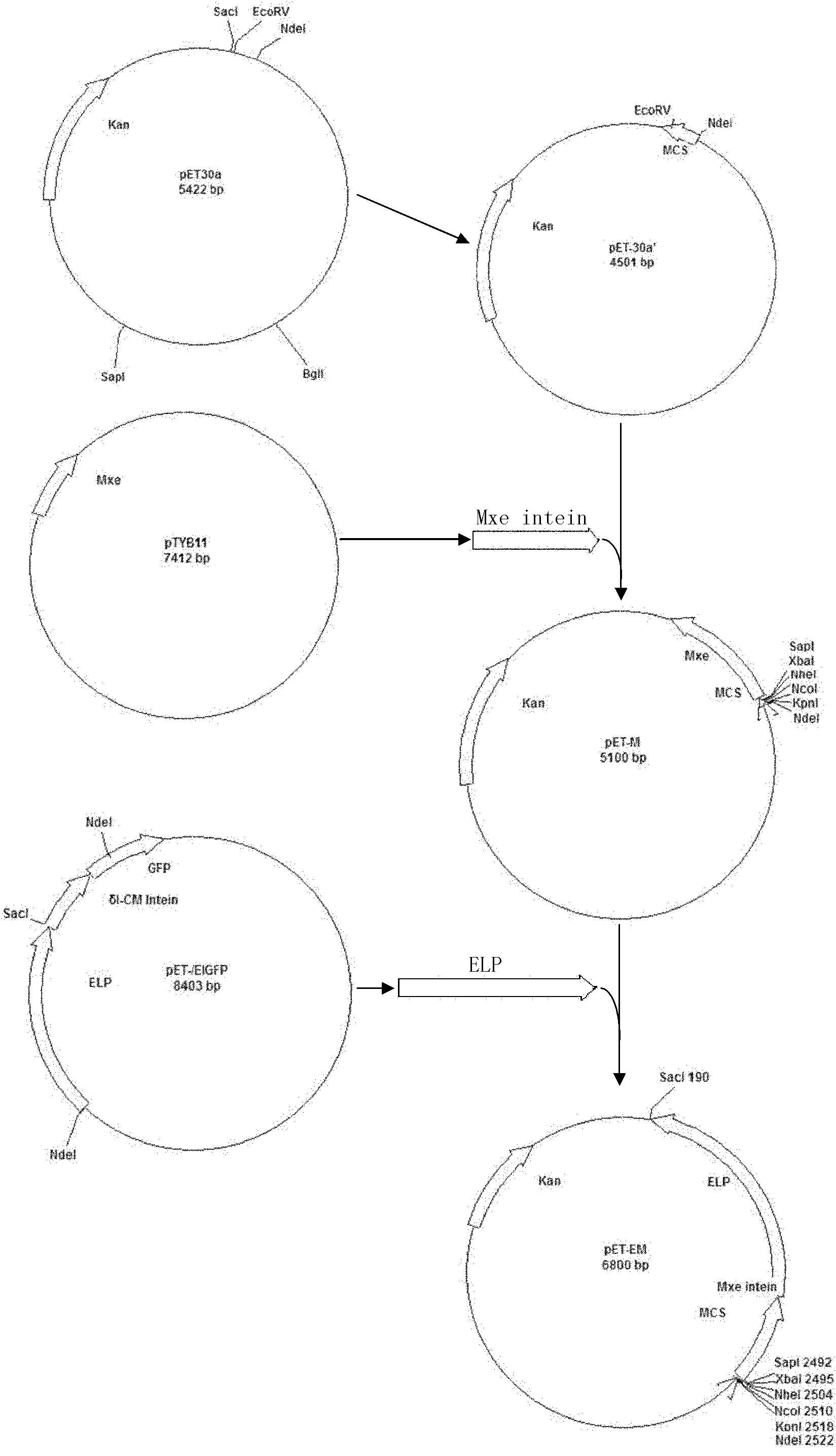
[0035]Example 1: Construction of pET-EM
[0036] The construction process of pET-EM is as follows figure 2 ,Specific steps are as follows:
[0037] 1) with restriction endonuclease SAP I and Bgl I digest the vector pET30a, separate the digested products by electrophoresis on 0.8% agarose gel, recover the large fragments digested by the DNA recovery kit in the gel, fill in the ends of the recovered products with Klenow large fragment DNA polymerase, and connect them with T4 DNA ligase. get none SAP The vector pET30a' of I restriction site;
[0038] 2) According to the Mxe on the commercial vector pYTB11 gyrA Intein coding sequence, design and synthesize a pair of primers:
[0039] P1: 5'-TCCATATGGGTACCCCATGGGCTAGCTCTAGAGGCTCTTCCTGCA
[0040] TCACGGGA-3' (SEQ ID NO.1) (introduced at the 5' end of the primer Nde I. Kpn I. Nco I. Nhe I. Xba I. SAP I restriction site);
[0041] P2: 5'-TT GATATC AGAGCGTGGCTGACGAACCCG-3' (SEQ ID NO.2) (the underline is intr...
Embodiment 2
[0044] Example 2: Prokaryotic expression and purification of African swine fever virus K205R gene
[0045] 1. Synthesize a pair of primers P1: 5'-GGTCATATGGTTGAGCCACGCGAACAG-3' (SEQ ID NO.3), P2: 5'-GGTTGCTCTTCCGCACTTCTTCATCATCTCTTTG-3' (SEQ ID NO.4), PCR method, from plasmid pGEX-4T-1 -K205R amplifies the K205R gene ( image 3 ), to recover the DNA fragment, the restriction enzyme Nde I and SAP Ⅰ After double digestion, insert the vector pET-EM to construct the recombinant expression vector pET-EM-K205R ( Figure 4 ).
[0046] 2. Transform the recombinant expression vector pET-EM-K205R into Escherichia coli BLR (DE3) competent cells, screen out positive recombinant bacteria, inoculate 50ug / ml kanamycin LB liquid medium, and place in a shaker (160rpm) at 37°C When the culture OD600 is 0.5, add IPTG to the final concentration of 0.4mM, induce at 20°C for 24h, precipitate the bacteria, and freeze repeatedly in 0.5ml lysis buffer (10mM Tris-HCl, 2mM EDTA, 0.1mg / ml Lysozyme,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com