Medical gargle product for Xerostomia and preparation method thereof
A technology for dry mouth and medical supplies, applied in the field of medicine, can solve the problems of no medical effect, expensive side effects of medicines, and impossibility of long-term use of medicines, etc., to assist natural healing mechanism, good broad-spectrum antiviral activity, and promote wound healing healing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] One. preparation total weight is the xerostomia medical supplies of 1000g containing semen solution:
[0031] a), take by weighing 1g carboxymethyl chitosan and be dissolved in 100g water for injection, obtain carboxymethyl chitosan solution;
[0032] b), weigh 1g lactoferrin, 0.1g immunoglobulin, 10g sorbitol, 1g methylparaben and 10g bovine colostrum extract respectively, dissolve them in 200g purified water, and obtain corresponding solutions after dissolving;
[0033] c), take by weighing 1g lysozyme and 0.1g lactoperoxidase respectively, be dissolved in 100g water for injection to obtain enzyme solution;
[0034] d), adding the remaining 575.8 g of water for injection into the solution prepared in steps a), b) and c) and stirring evenly, subpackaging and sterilizing at 85° C. for 15 minutes to obtain a finished product.
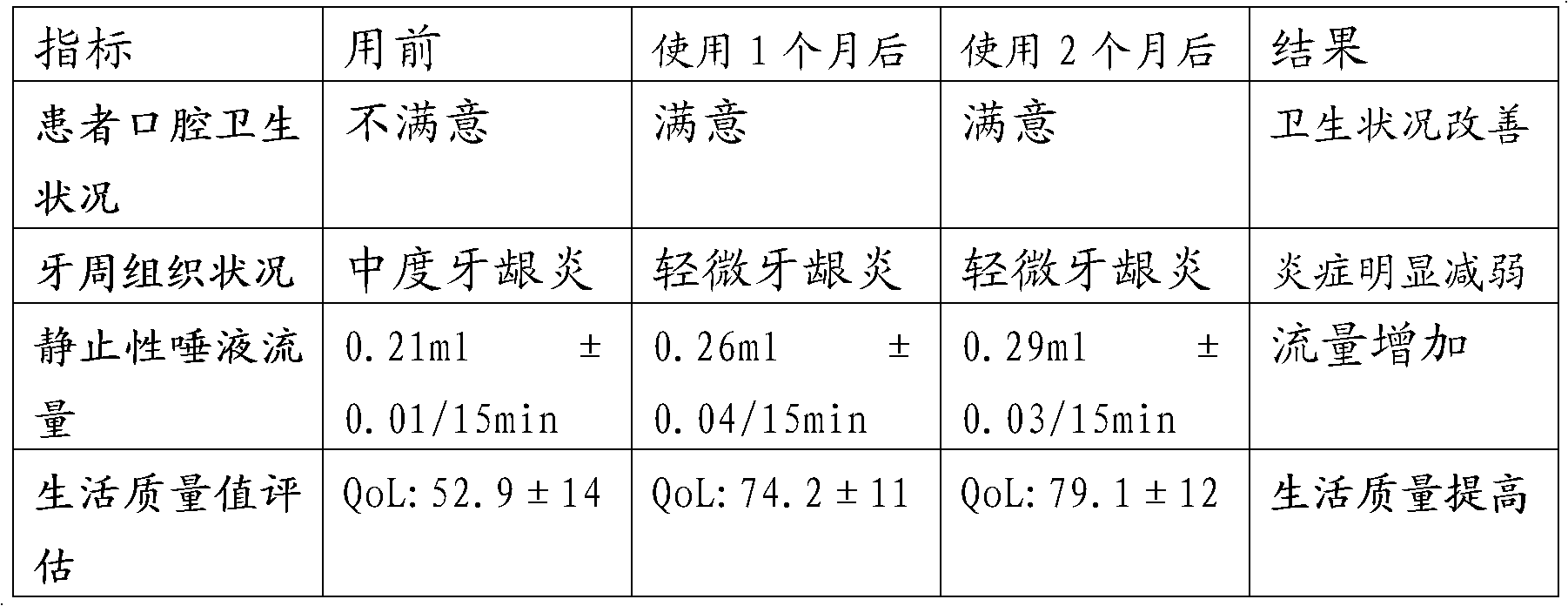
[0035] Two. Example 1 xerostomia-containing solution finished product effect detection
[0036] 1), the specific parameters of the xerostomia-c...
Embodiment 2
[0046] One. preparation total weight is the xerostomia medical supplies of 1000g containing semen solution:
[0047] a), take by weighing 50g hydroxyethyl chitosan and dissolve in 100g water for injection to obtain hydroxyethyl chitosan solution;
[0048] b), take by weighing 10g lactoferrin, 0.5g immunoglobulin, 50g xylitol, 10g ethylparaben and 100g bovine colostrum extract respectively, dissolve in 200g purified water, obtain corresponding solution after dissolving;
[0049] c), take by weighing 5g lysozyme and 0.5g lactoperoxidase respectively, be dissolved in 100g water for injection to obtain enzyme solution;
[0050] d), adding the remaining 374 g of water for injection into the solution prepared in steps a), b) and c), and stirring evenly, subpackaged and sterilized at 85° C. for 15 minutes to obtain a finished product.
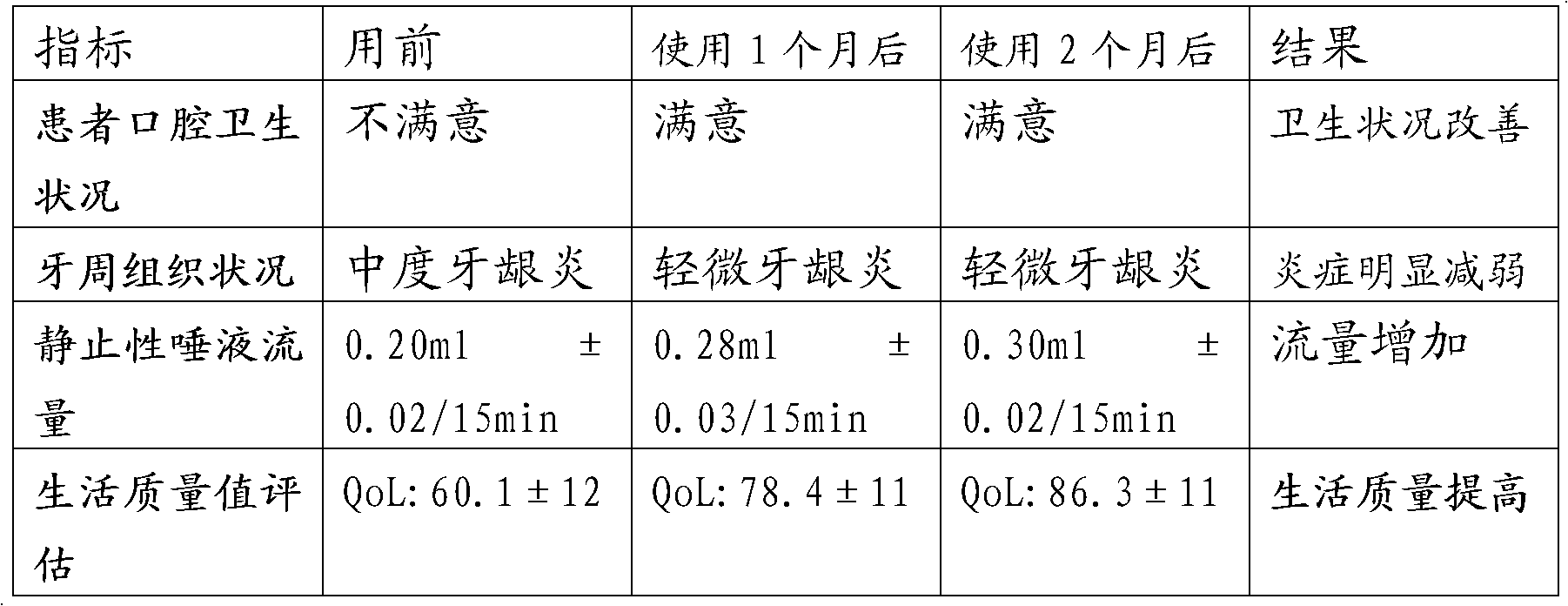
[0051] Two. Example 2 xerostomia containing liquid medical supplies effect detection
[0052]1), the specific parameters of the xerostomia containi...
Embodiment 3
[0062] One. preparation total weight is the xerostomia medical supplies of 1000g containing semen solution:
[0063] a), take by weighing 20g hydroxypropyl chitosan and be dissolved in 100g water for injection, obtain hydroxypropyl chitosan solution;
[0064] b), weigh 5g lactoferrin, 1g immunoglobulin, 100g maltitol, 5g propylparaben and 50g bovine colostrum extract respectively, dissolve them in 200g purified water, and obtain corresponding solutions after dissolving;
[0065] c), take by weighing 2g lysozyme and 5g lactoperoxidase respectively, be dissolved in 100g water for injection to obtain enzyme solution;
[0066] d), adding the remaining 412 g of water for injection into the solution prepared in steps a), b) and c), and stirring evenly, subpackaged and sterilized at 85° C. for 15 minutes to obtain a finished product.
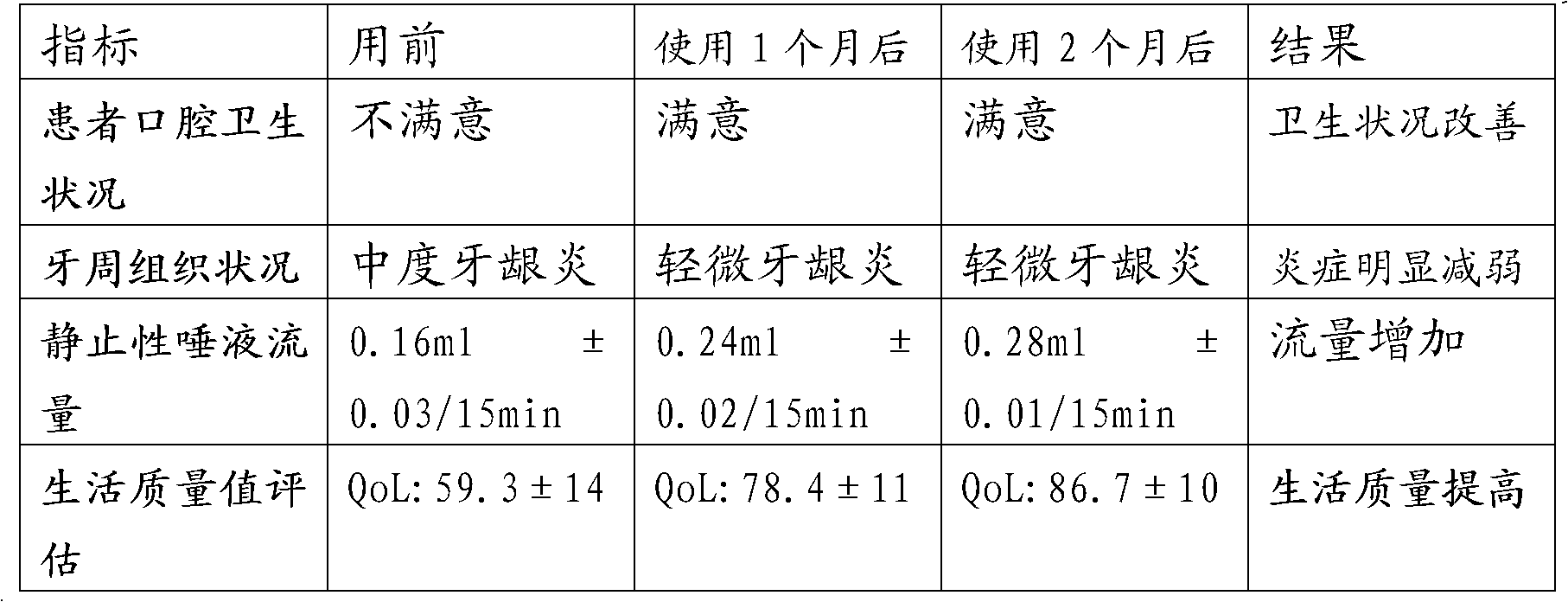
[0067] Two. Example 3 xerostomia containing liquid medical supplies effect detection
[0068] 1), the specific parameters of the xerostomia containi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com