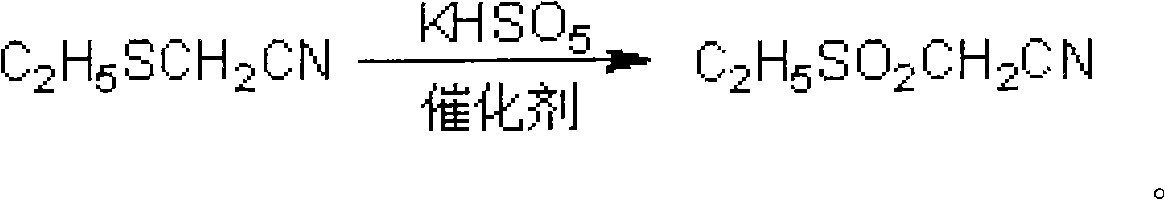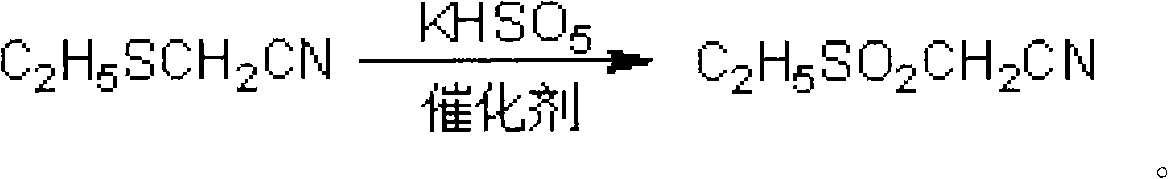Method for preparing ethylsulfonyl acetonitrile
A technology of ethylsulfonylacetonitrile and ethylthioacetonitrile, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve problems such as multiple organic solvents, difficult waste liquid treatment, etc., and achieves easy operation and product yield. The effect of high rate and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Add 10.1g (0.10mol) ethylthioacetonitrile to a 1000mL three-necked flask, stir at room temperature, and dropwise add an aqueous solution of potassium monopersulfate complex tetrabutylammonium bromide (92.1g potassium monopersulfate complex Compound and 9.7g tetrabutylammonium bromide were dissolved in 500mL of water, containing active ingredients potassium monopersulfate 0.30mol, tetrabutylammonium bromide 0.03mol), 1h dripped. Stir at below 40°C for 2h, add 100mL chloroform and extract twice. The organic layer was taken and the solvent was evaporated to obtain 12.1 g of the product with a yield of 91.0% and a content of 98.9% (quantitative analysis by gas chromatography). The NMR data of the product obtained: 1 HNMR (CDCl 3 )δ: (ppm) 1.50-1.55 (t, 3H, -CH 2 CH 3 ), 3.30-3.37 (q, 2H, -CH 2 CH 3 ), 3.97-3.98 (m, 2H, -CH 2 CN).
Embodiment 2
[0019] Example 2 Add 10.1g (0.10mol) ethylthioacetonitrile to a 1000mL three-necked flask, stir at room temperature, and dropwise add an aqueous solution of potassium monopersulfate complex-benzyltriethylammonium chloride (61.5g monopersulfate Potassium hydrogen complex and 2.3g of benzyltriethylammonium chloride were dissolved in 400mL of water, containing active ingredients potassium monopersulfate 0.20mol, benzyltriethylammonium chloride 0.01mol), dripping over 1.5h. Stir at below 20°C for 1.5h, add 100mL chloroform and extract twice. The organic layer was taken and the solvent was evaporated to obtain 12.0 g of the product with a yield of 90.2% and a content of 97.7% (quantitative analysis by gas chromatography).
Embodiment 3
[0020] Example 3 Add 10.1g (0.10mol) ethylthioacetonitrile to a 2000mL three-necked flask, stir at room temperature, and dropwise add an aqueous solution of potassium monopersulfate complex-tetrabutylammonium bromide (123.0g potassium monopersulfate The complex and 16.1g of tetrabutylammonium bromide were dissolved in 800mL of water, containing active ingredients potassium monopersulfate 0.40mol, tetrabutylammonium bromide 0.05mol), and dripped for 1 hour. Stir at below 50°C for 4h, add 100mL ethyl acetate to extract twice. The organic layer was taken and the solvent was evaporated to obtain 12.1 g of the product with a yield of 91.0% and a content of 97.8% (quantitative analysis by gas chromatography).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com