Method for preparing submicron lamellar magnesium hydroxide by intensifying alkali
A magnesium hydroxide, sub-micron technology, applied in the direction of magnesium hydroxide, etc., can solve the problems that the crystallinity and shape of magnesium hydroxide are difficult to meet the requirements of flake magnesium hydroxide flame retardants, and the cost of magnesium hydroxide increases. Achieve the effect of reducing the activation energy of hydrolysis reaction, saving the alkali used for precipitation, and shortening the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
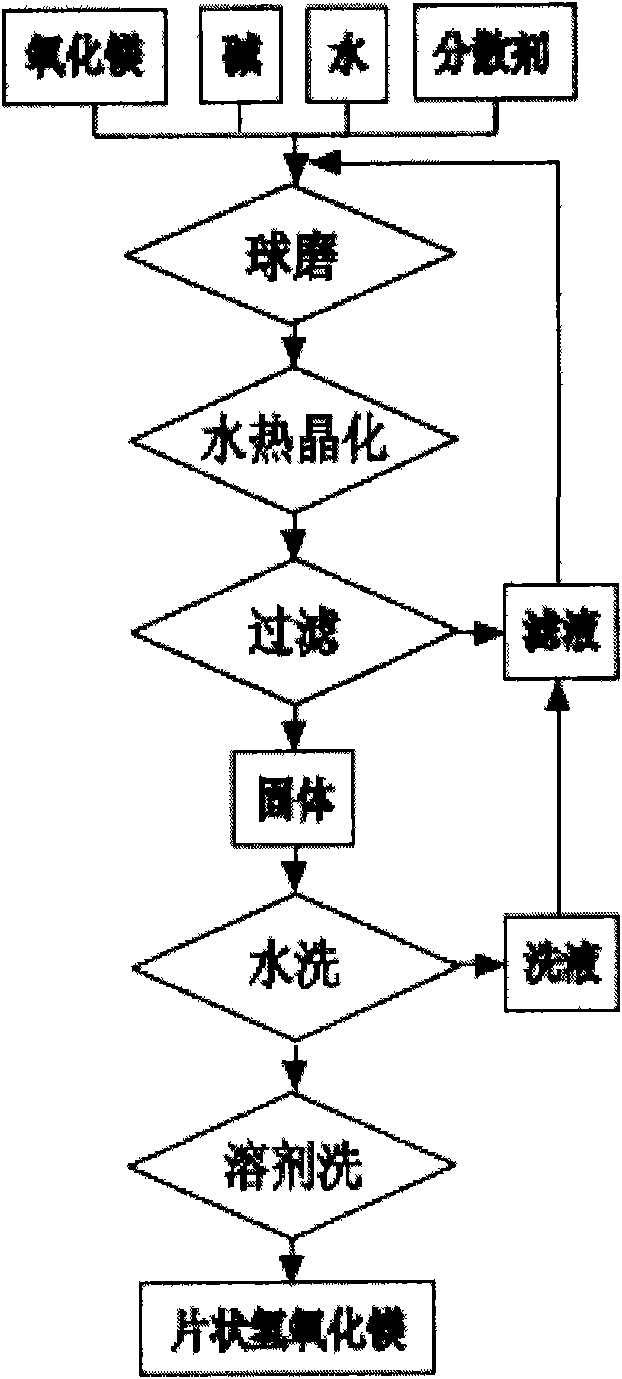
[0042] The composition of magnesium oxide is that the content of MgO is 98.5%, and the loss on ignition is less than 4.5%.
[0043] Mix the above-mentioned magnesium oxide powder 20.2g, sodium hydroxide 10g, 0.5g polyvinylpyrrolidone and 100.0 water;
[0044] Transfer to a 500ml nylon ball mill jar, and use 100g of reburned magnesium oxide grinding balls with a diameter of 6mm for 4h at a speed of 400r / min to form a slurry to be crystallized.
[0045] Pour the above-mentioned slurry to be crystallized into a 200ml pressure volume bomb lined with Teflon, and crystallize at 200°C for 4 hours (h);
[0046] After the above reaction, the slurry was suction filtered at room temperature, washed three times with deionized water, washed once with industrial ethanol, and dried in an oven at 120° C. for 3 hours to obtain flaky magnesium hydroxide.
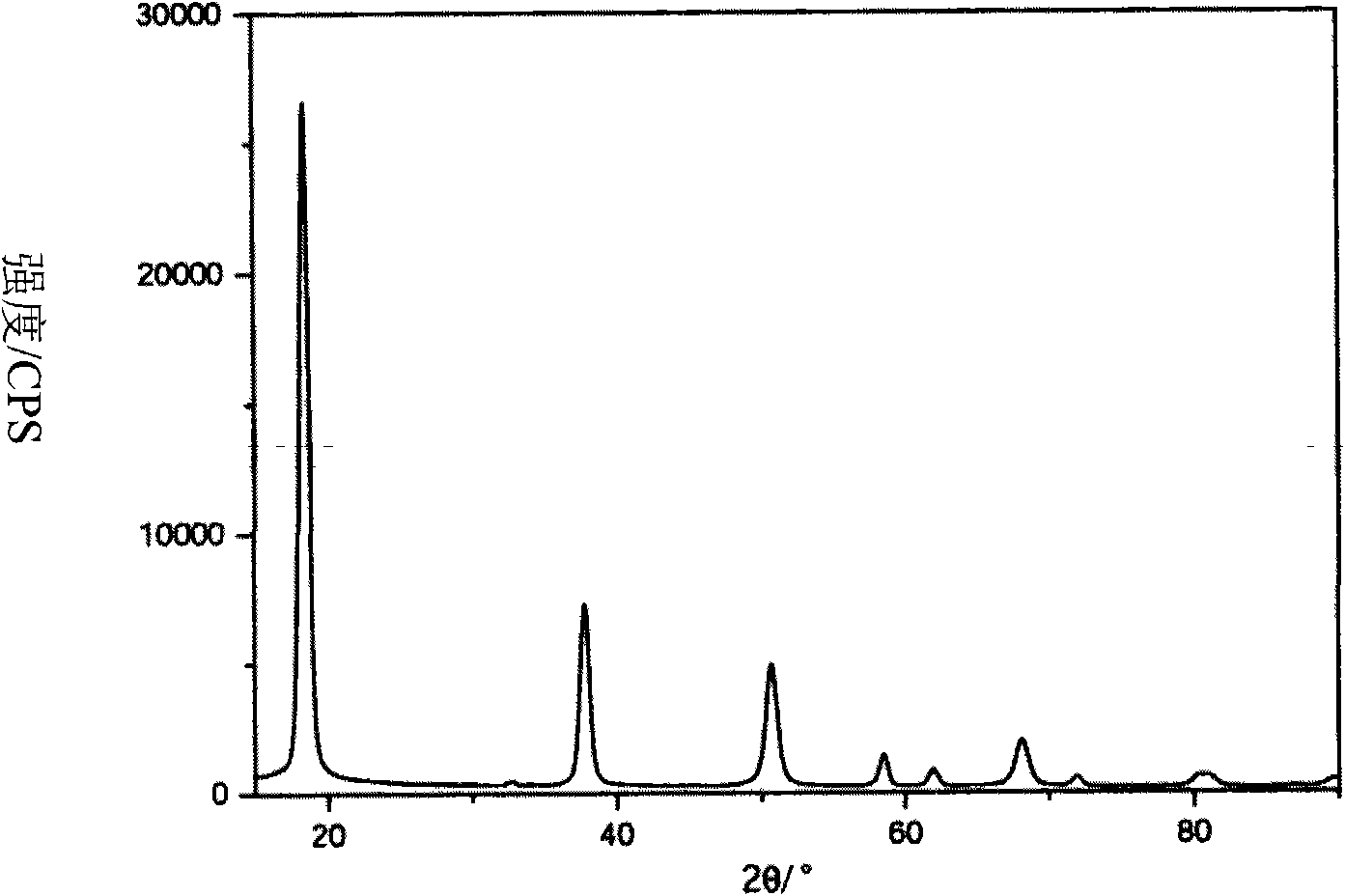
[0047] The XRD diffraction pattern of the obtained magnesium hydroxide structure is as follows figure 2 shown, the appearance is as ima...
Embodiment 2
[0049]Mix above-mentioned magnesium oxide powder 1kg, ammonia water 200ml, 5g polyvinyl alcohol and 5kg water;
[0050] Transfer to SX-30 type stirring ball mill, use 5kg diameter 6mm reburned magnesia grinding ball for 8h, power is 2kw, form the slurry to be crystallized.
[0051] Transfer the above slurry to be crystallized into a 10L stirring reaction kettle, and crystallize at 160°C for 10h,
[0052] After the above reaction, the slurry was suction filtered at room temperature, washed three times with deionized water, washed once with industrial ethanol, and dried in an oven at 120° C. for 3 hours to obtain flaky magnesium hydroxide.
Embodiment 3
[0054] Mix the above-mentioned magnesium oxide powder 20.2g, diethylamine 10g, 0.5g polyvinylpyrrolidone and 100.0 water;
[0055] Transfer to a 500ml nylon ball mill jar, and use 100g of reburned magnesium oxide grinding balls with a diameter of 6mm for 4h at a speed of 400r / min to form a slurry to be crystallized.
[0056] Pour the above slurry to be crystallized into a 200ml pressure volume bomb lined with PTFE, and crystallize at 200°C for 4h;
[0057] After the above reaction, the slurry was suction filtered at room temperature, washed three times with deionized water, washed once with industrial ethanol, and dried in an oven at 120° C. for 3 hours to obtain flaky magnesium hydroxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com