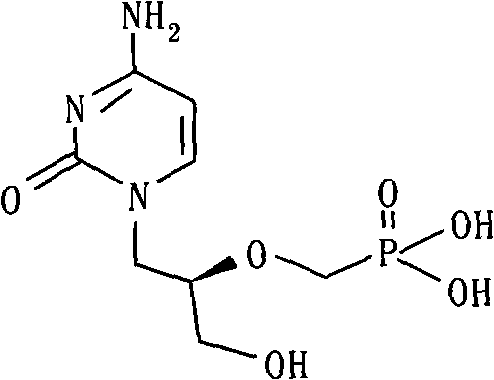
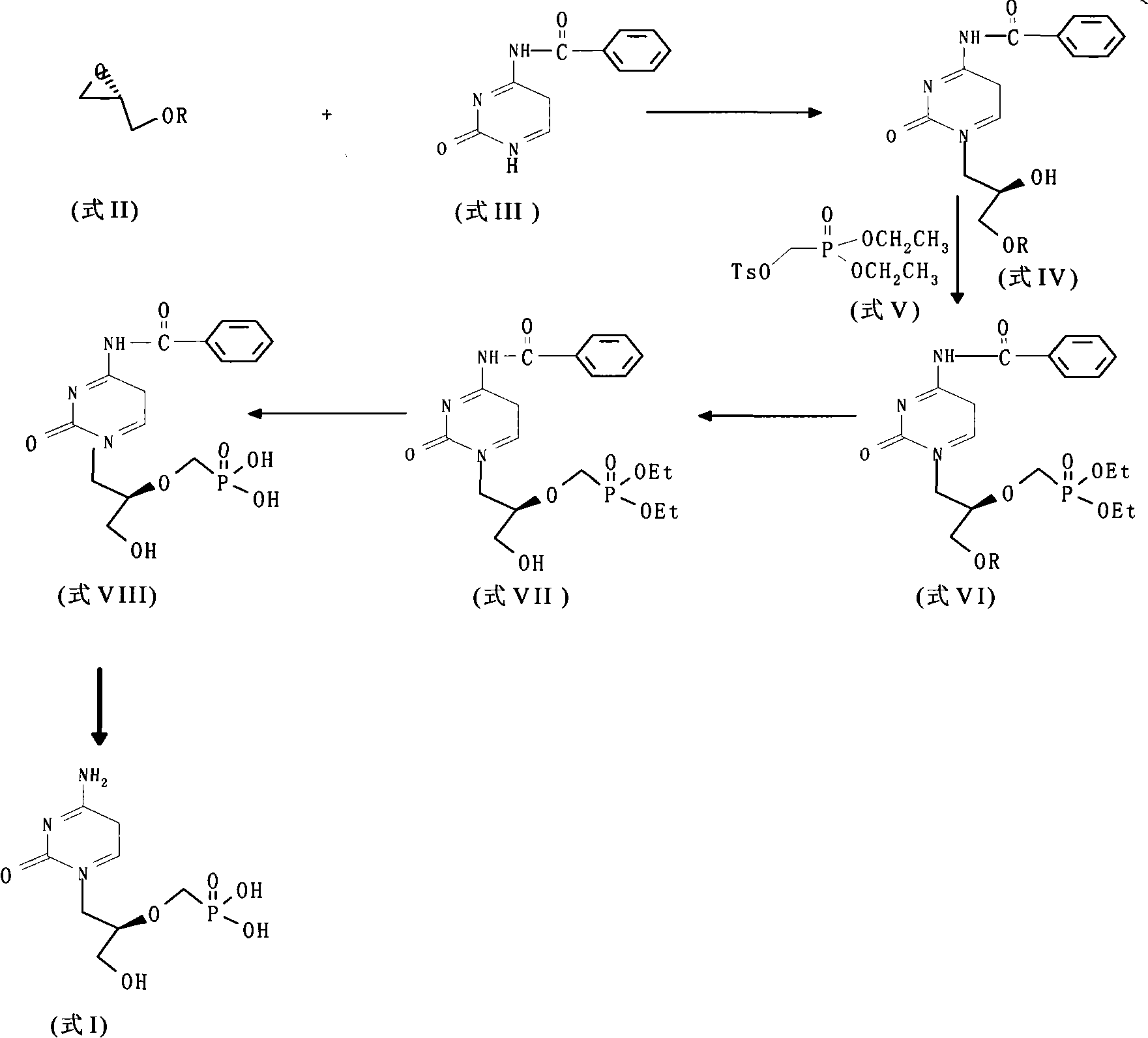
Method for preparing p-tosyloxymethyl diethyl phosphonate
A technology of toluenesulfonyloxymethyl diethyl phosphate and p-toluenesulfonyl chloride, which is applied in the field of cidofovir intermediates, can solve the problems of difficult liquid separation process, product loss and high cost, and achieves correct product structure, The effect of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In the reaction tank, add 60kg (437mol) of diethyl phosphite, 15kg (500mol) of paraformaldehyde and 101kg (1000mol) of triethylamine, and heat the above reaction mixture to 90°C under stirring. Within 30 minutes, the white slurry After 3 hours, the reaction mixture was cooled to room temperature; after adding 200 kg of DMF, it was cooled to 0° C., 96 g (500 mol) of p-toluenesulfonyl chloride was added within 5 minutes, the reaction mixture was stirred at room temperature for 16 hours, and ethyl acetate was added. 200L, filter the insoluble matter, wash the filtrate with saturated sodium bicarbonate, water and saturated sodium chloride (200L) respectively, dry over anhydrous magnesium sulfate, filter, concentrate the filtrate, add toluene (2×250ml) to the residue and concentrate under reduced pressure, Obtained 106 kg of diethyl p-toluenesulfonyloxymethyl phosphate in the form of clear yellow oil, with a yield of 75%.
[0019] The proton nuclear magnetic spectrum data of...
Embodiment 2
[0022] In the reaction tank, add 60 kg (437 mol) of diethyl phosphite, 5 kg (166.7 mol) of paraformaldehyde and 101 kg (1000 mol) of triethylamine, and heat the above reaction mixture to 110 ° C under stirring. Within 30 minutes, a white slurry The solid becomes a clear solution, the temperature is lowered to 80°C, then 10kg (333mol) of paraformaldehyde is added, heated to 110°C under stirring, within 10 minutes, the white slurry turns into a clear solution, after 3 hours the reaction The mixture was cooled to room temperature; after adding 200 kg of DMF, it was cooled to 0°C, 96 g (500 mol) of p-toluenesulfonyl chloride was added within 5 minutes, the reaction mixture was stirred at room temperature for 16 hours, 200 L of ethyl acetate was added, the insoluble matter was filtered off, and the filtrate was washed with saturated Wash with sodium bicarbonate, water and saturated sodium chloride (200L), dry over anhydrous magnesium sulfate, filter, concentrate the filtrate, add to...
Embodiment 3
[0024] In the reaction tank, add 60kg (437mol) of diethyl phosphite, 15kg (500mol) of paraformaldehyde and 101kg (1000mol) of triethylamine, and heat the above reaction mixture to 110°C under stirring. Within 30 minutes, the white slurry After 3 hours, the reaction mixture was cooled to room temperature; after adding 200 kg of tetrahydrofuran, it was cooled to 0° C., and 96 g (500 mol) of p-toluenesulfonyl chloride was added within 5 minutes; the reaction mixture was stirred at room temperature for 16 hours, and ethyl acetate was added. Ester 200L, filter out insoluble matter, wash the filtrate with saturated sodium bicarbonate, water and saturated sodium chloride (200L) respectively, dry over anhydrous magnesium sulfate, filter, concentrate the filtrate, add toluene (2×250ml) to the residue and concentrate under reduced pressure , to obtain 100 kg of diethyl p-toluenesulfonyloxymethyl phosphate in the form of clear yellow oil, with a yield of 71%. The structure of the product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com