Epoxy-group-containing copolymer, epoxy (methyl) acrylate copolymer using same, and processes for producing copolymers
A manufacturing method and acrylate technology, applied in plastic/resin/wax insulators, organic insulators, etc., can solve the reduction of mechanical properties such as the strength and elongation of the cured product, the reduction of the strength and elongation of the cured product, and the chemical structure. Low molecular weight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
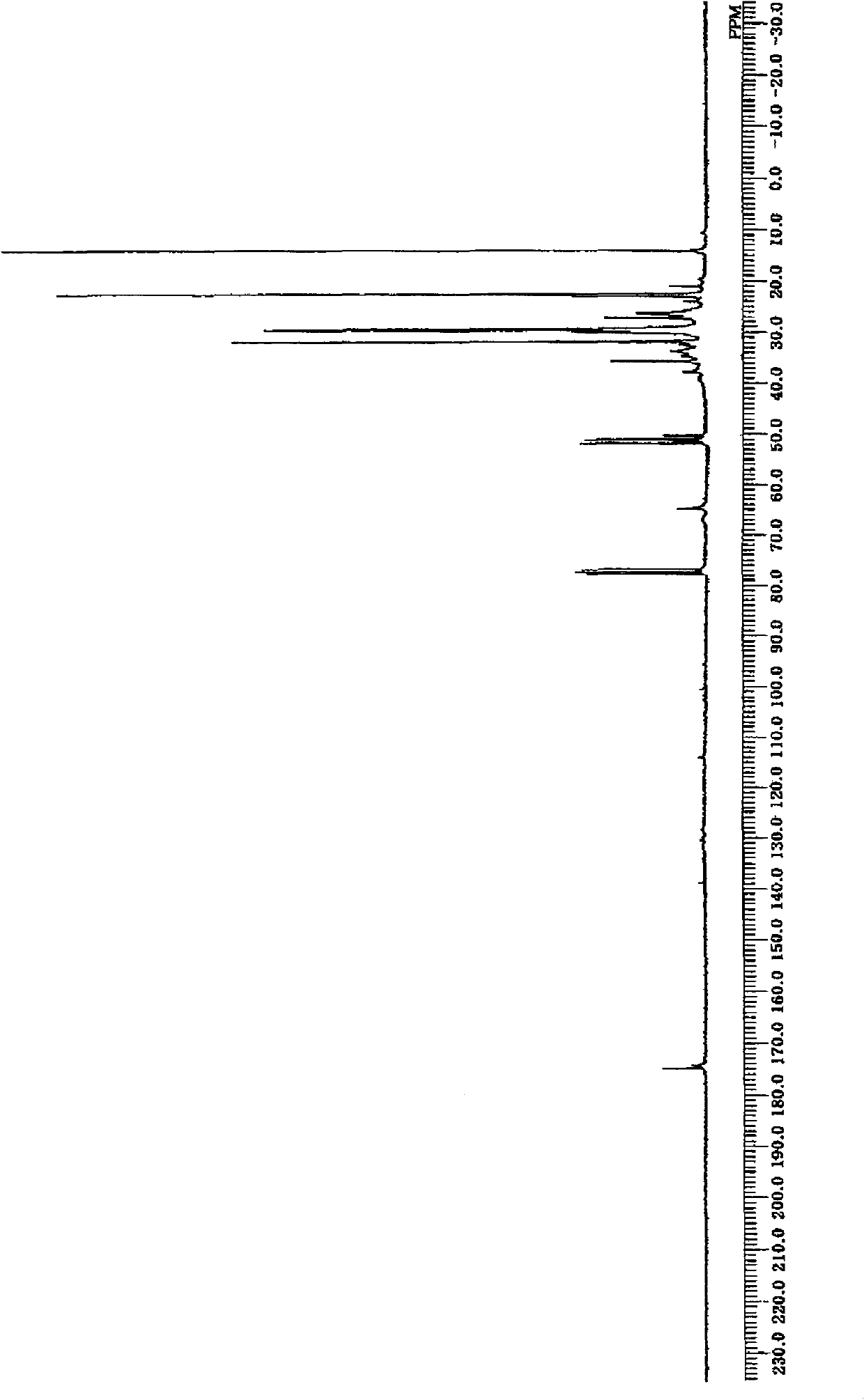
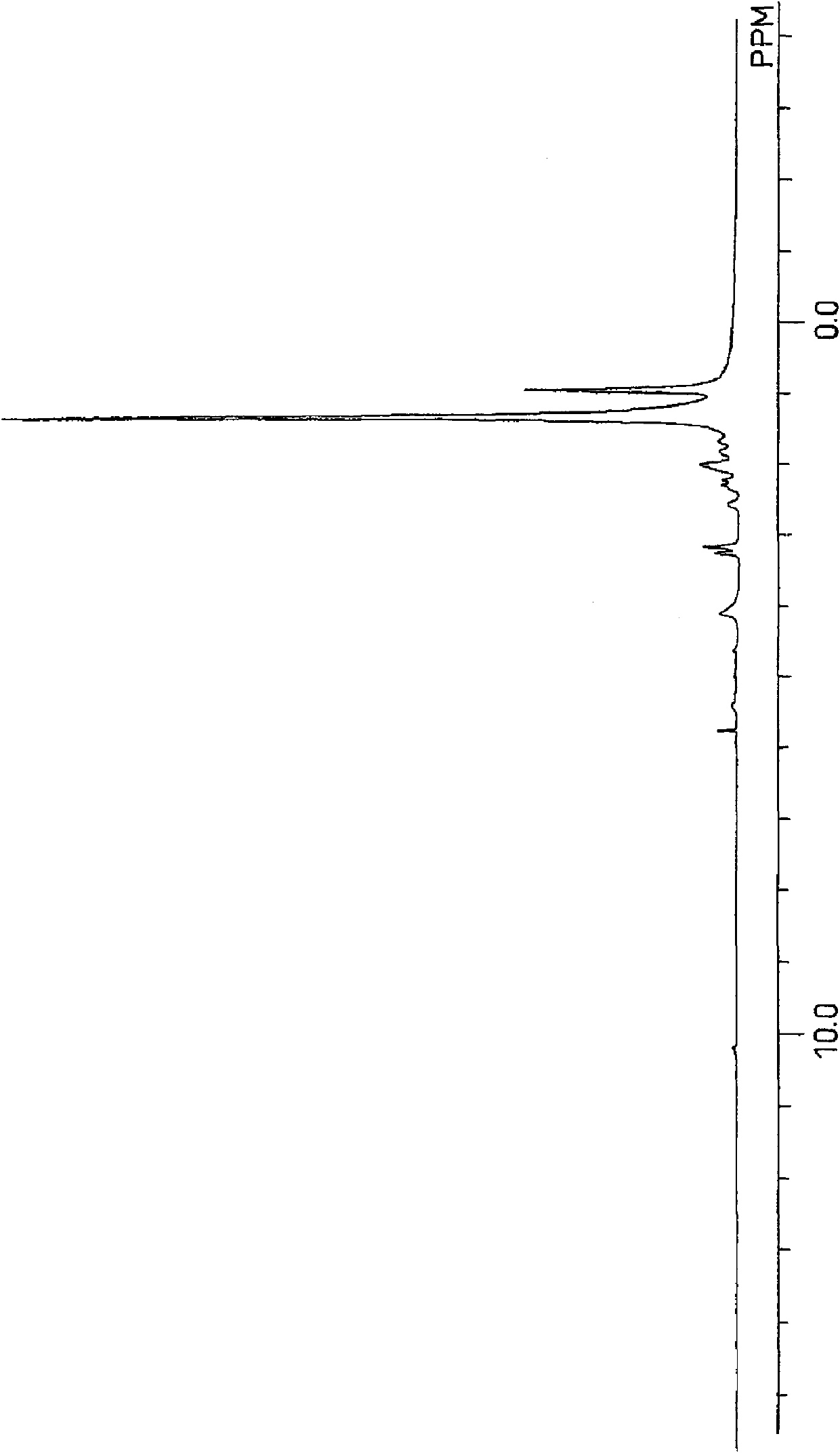
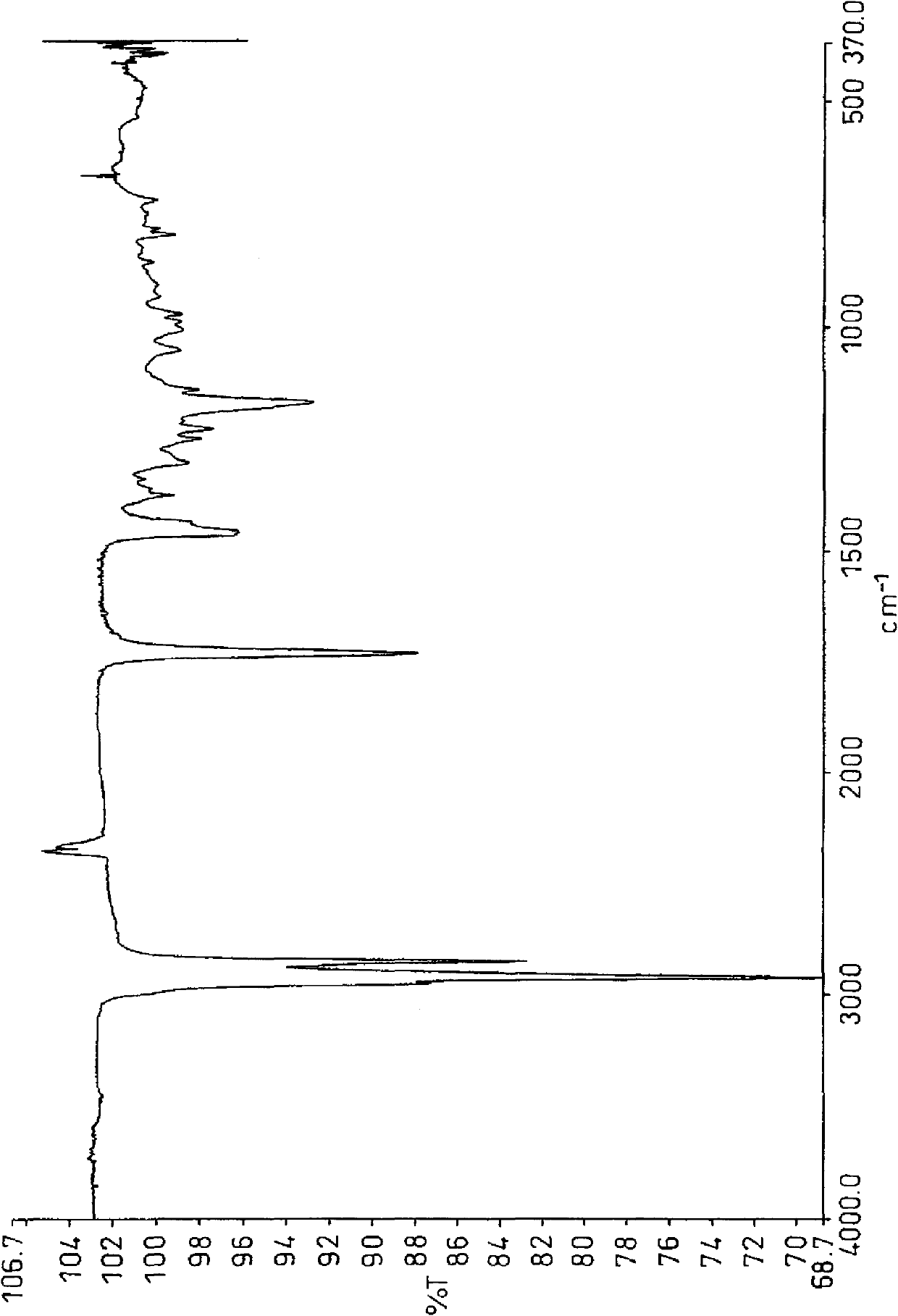
[0167]In the PPV-4060 personalized organic synthesis device (simple autoclave) of Tokyo Physical and Chemical Machinery, add 3,4-epoxycyclohexane-1-allyl carboxylate (hereinafter referred to as "CEA") 9.11g (50mmol ), 36.6 g of 1-decene (Linialen-10, manufactured by Idemitsu Kosan Co., Ltd., purity 96.6% 250 mmol), 2.238 g of di-tert-butyl peroxide (Parbuchol-D, manufactured by NOF Corporation, purity 98%, 15 mmol), and after nitrogen replacement, the reaction vessel was sealed and reacted at 144° C. for 3 hours. After the reaction, use the 6850 series II gas chromatograph (GC) produced by Agilent Technologies to measure the residual amount of monomers. As a result, 55.8% of allyl 3,4-epoxycyclohexane-1-carboxylate has reacted, 1-decane 25.4% of ene was reacted (the copolymerization ratio is 1:2.28). GPC analysis showed that the number average molecular weight was 1230 and the weight average molecular weight was 1980. In addition, CEA used as a raw material was manufactured b...
Embodiment 2~24
[0173] Use various epoxy-containing monomers (the first monomer) and olefins (the second monomer), change the polymerization conditions, and polymerize under the same condition that the feed weight is about 50g as in Example 1, the obtained results together with The results of Example 1 are shown in Table 1 below. It was found that copolymerization proceeded under any conditions.
[0174] [Table 1]
[0175]
[0176] パヘキサ-TMH: 1,1-(di-tert-hexylperoxide)-3,3,5-trimethylcyclohexane
[0177] [Evaluation of Thermosetting Composition for Cover Layer]
[0178]
Embodiment 25
[0180] Components other than the fluorine-based surfactant and the leveling agent among the components shown below were blended with the copolymer obtained in Example 1, and kneaded using triple rollers. After kneading with 3 rollers, add a surfactant and a leveling agent, and mix with a resin mixer to prepare a thermosetting resin composition.
[0181] Copolymer of Example 1: 28.6 parts by mass
[0182] Acid anhydride: HN-5500E (manufactured by Hitachi Chemical Industries, Ltd.) 8.4 parts by mass
[0183] Curing agent: 0.37 parts by mass of 2E4MZ (manufactured by Shikoku Chemical Industry Co., Ltd.)
[0184] Thixotropic agent: Aerosil R974 (manufactured by Japan Aerosil Co., Ltd.) 1.85 parts by mass
[0185] Barium sulfate: 7.4 parts by mass of B94 (manufactured by Sakai Chemical Industry Co., Ltd.)
[0186] Silicone powder: 1.85 parts by mass of X-52-854 (manufactured by Shin-Etsu Chemical Co., Ltd.)
[0187] Fluorinated surfactant: 0.11 parts by mass of PolyFOX PF6520 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com