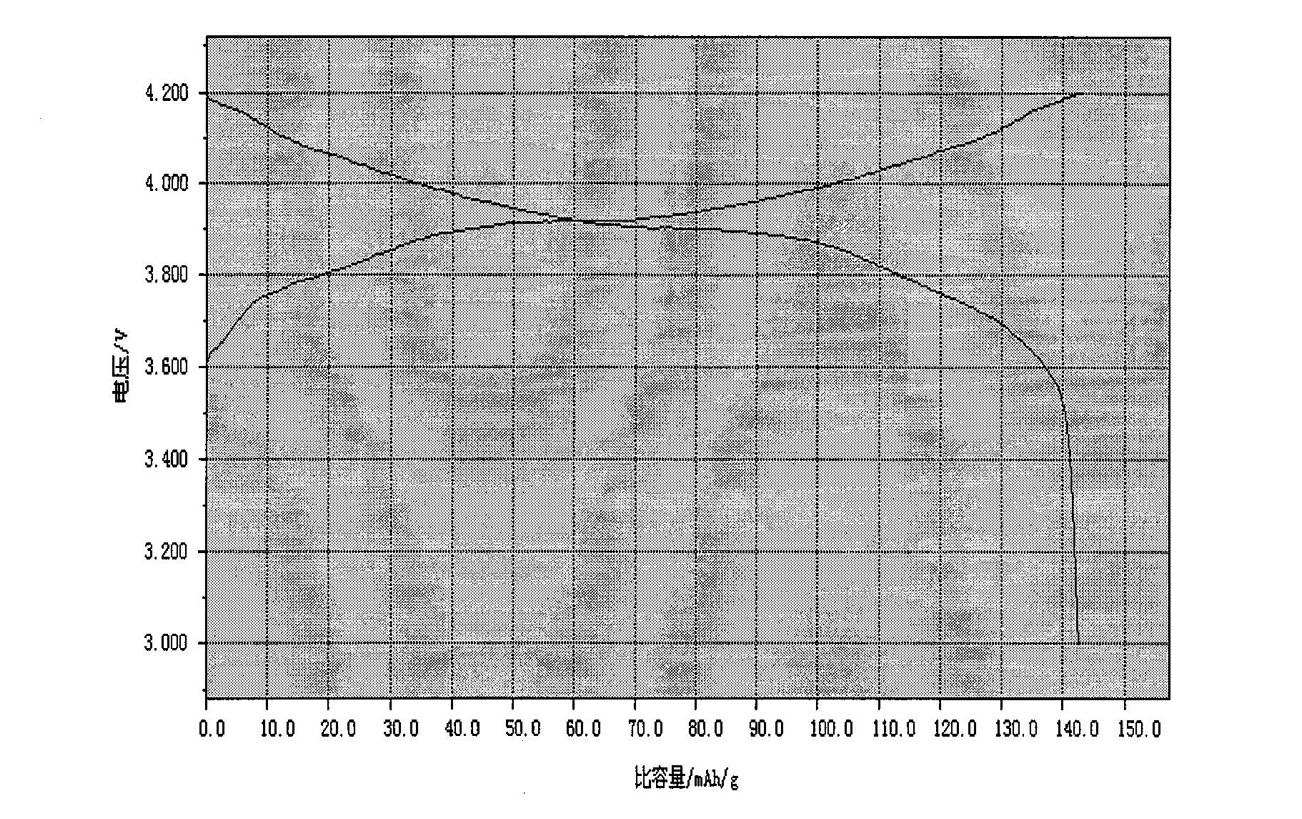
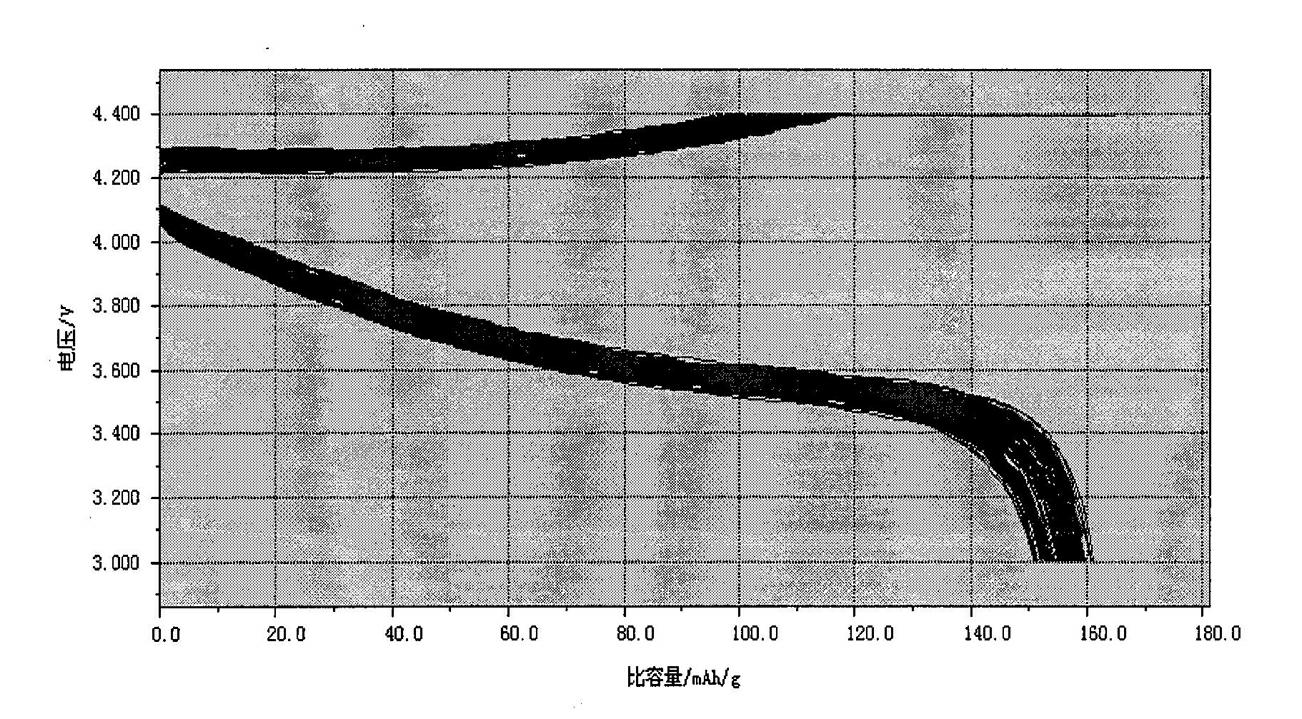
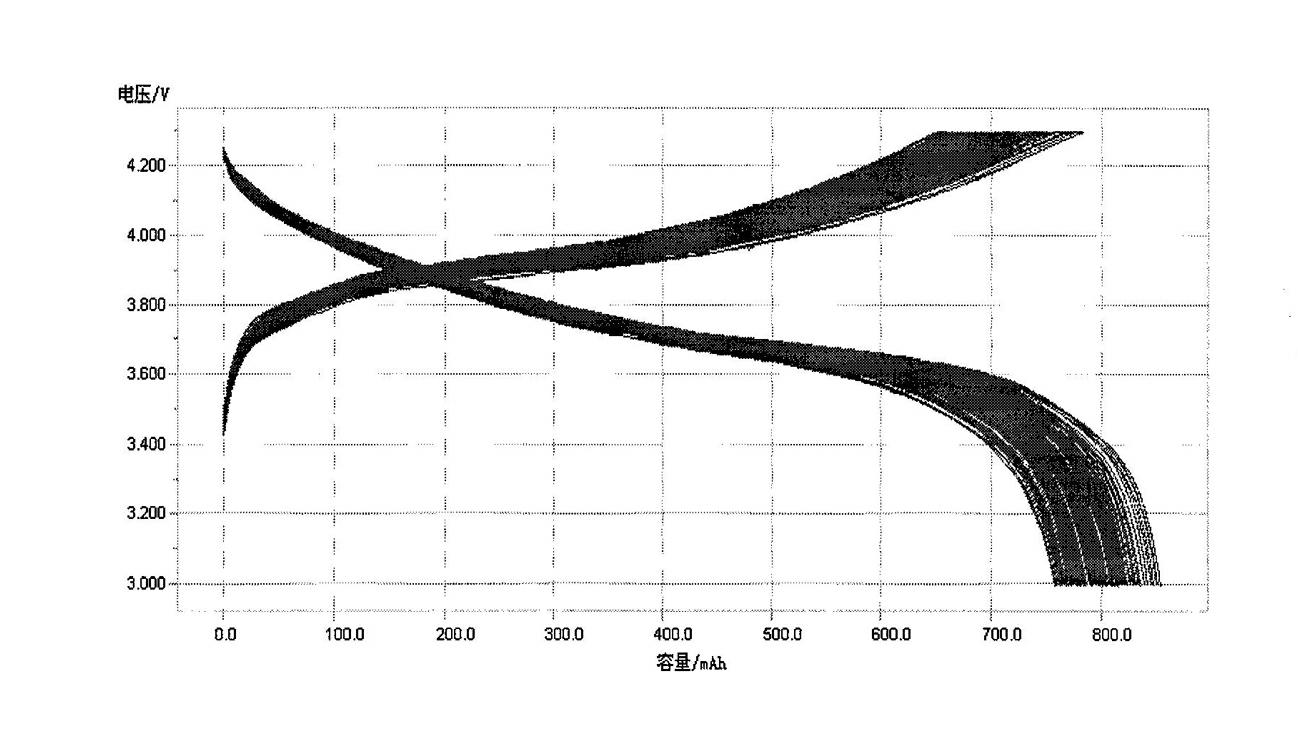
Method for preparing high-compaction high-voltage lithium cobaltite cathode material
A positive electrode material, high-voltage technology, applied in battery electrodes, circuits, electrical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Mix 10L of the configured 5mol / L nickel sulfate solution and 30L of the configured 5mol / L manganese sulfate solution in a 500L reactor, and slowly add the 5mol / L complex with a peristaltic pump under vigorous stirring. Mixture of ammonia solution 10L. Stir for 1 hour to mix well and form a complex nickel-manganese solution. Slowly add 192.64Kg of tricobalt tetroxide and stir for 1 hour. After the tricobalt tetroxide is completely infiltrated, use a peristaltic pump to add 5mol / L sodium hydroxide solution at a uniform speed until the pH of the solution is 11 and stop adding. Continue stirring and aging for 24 hours, control the reaction temperature at 50°C, and the stirring speed at 500r / min to obtain [Ni 0.5 mn 1.5 (OH) 4 ] 0.2 (Co 3 o 4 ) 0.8 The precursor was washed with deionized water until the pH value was 8, dried in a drying oven at 100°C, and sintered at 500°C to obtain the oxide of the precursor. Lithium carbonate is added according to the ratio of lith...
Embodiment 2
[0027]Mix 50L of the configured 5mol / L nickel sulfate solution and 50L of the configured 5mol / L manganese sulfate solution in a 500L reactor, and slowly add the 5mol / L complex with a peristaltic pump under vigorous stirring. Mixture ammonia solution 15L. Stir for 1 hour to mix well and form a complex nickel-manganese solution. Slowly add 280.77Kg of tricobalt tetroxide and stir for 1 hour. After the tricobalt tetroxide is completely infiltrated, use a peristaltic pump to add 5mol / L sodium hydroxide solution at a uniform speed until the pH of the solution is 11 and stop adding. Continue stirring and aging for 24 hours, control the reaction temperature at 50°C, and the stirring speed at 500r / min to obtain [Ni 0.5 mn 0.5 (OH) 2 ] 0.3 (Co 3 o 4 ) 0.7 The precursor was washed with deionized water until the pH value was 8, dried in a drying oven at 120°C, and sintered at 500°C to obtain the oxide of the precursor. Lithium carbonate is added according to the ratio of lithium ...
Embodiment 3
[0029] Mix 50L of the configured 3mol / L nickel sulfate solution and 50L of the configured 2mol / L manganese sulfate solution in a 500L reaction kettle, and slowly add 1mol / L of the complex with a peristaltic pump under vigorous stirring. Mixture ammonia solution 20L. Stir for 1 hour to mix well and form a complex nickel-manganese solution. Slowly add 240.80Kg of tricobalt tetroxide and stir for 1 hour. After the tricobalt tetroxide is completely infiltrated, use a peristaltic pump to add 5mol / L sodium hydroxide solution at a uniform speed until the pH of the solution is 12 and stop adding. Continue stirring and aging for 24 hours, control the reaction temperature at 50°C, and the stirring speed at 600r / min to obtain [Ni 0.6 mn 0.4 (OH) 2 ] 0.2 (Co 3 o 4 ) 0.8 The precursor was washed with deionized water until the pH value was 8, dried in a drying oven at 120°C, and sintered at 450°C to obtain the oxide of the precursor. Lithium carbonate is added according to the ratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Compaction density | aaaaa | aaaaa |
| Compaction density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com