Method for preparing recyclable chiral catalyst and application thereof
A chiral catalyst and solution technology, applied in the field of chiral catalyst preparation, can solve the problems of non-recyclable reaction cost burden, reduce catalyst catalytic activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Preparation of proline-loaded nanofiber membrane catalyst
[0014] 10g of acetic acid solution with 7% polyvinyl alcohol content and 0.07g of L-proline were stirred evenly, then put into a syringe, and electrospun to form a fiber membrane with nanostructure, the spinning voltage was 10 kV, and the receiving distance was 15cm. Then the membrane was vacuum-dried at 60° C. to obtain a proline-loaded nanofiber membrane, and then the obtained membrane was stirred in triethylamine-dichloromethane solution for half an hour, and then vacuum-dried at 70° C. to obtain a catalyst.
Embodiment 2
[0015] Embodiment 2: the condensation reaction of catalytic 4-cyanobenzaldehyde and acetone
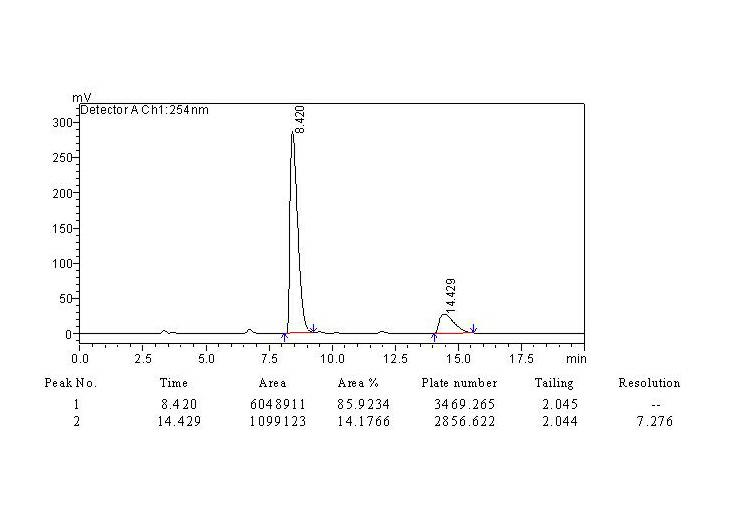
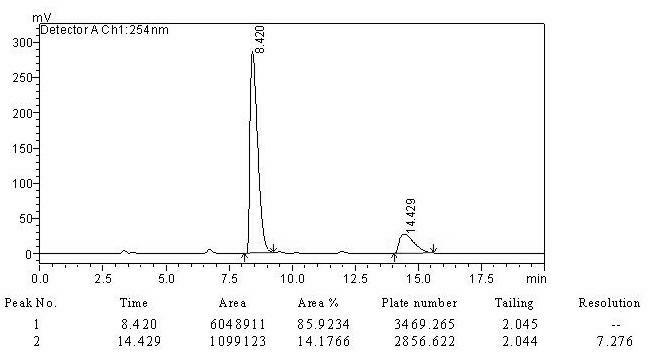
[0016] 4-Cyanobenzaldehyde (1mmol, 131mg) was dissolved in acetone (5mL), and 0.25g of proline membrane catalyst was added, and stirred at room temperature until the aldehyde was completely reacted. with saturated NH 4 Cl dilution, ethyl acetate extraction. The organic layer was dried over anhydrous magnesium sulfate, and then evaporated to remove the solvent. Column chromatography gave the product (4R)-4-(4'-cyanophenyl)-4-hydroxyl-2-butanone, with a yield of 88.2% and an ee value of 71.8%.
Embodiment 3
[0017] Embodiment 3: the recycling of catalyst:
[0018] By cleaning the catalyst after catalyst reaction recovery in Example 2 with acetone, drying, and reusing 3 times after activation, its catalytic activity is detected as shown in Table 1. As can be seen from Table 1, the proline-loaded proline of the present invention After the nanofiber membrane catalyst is reused 3 times, its catalytic efficiency is basically not reduced. The specific data is shown in the table below.
[0019] Table 1: Statistical Table of Catalytic Efficiency after Catalyst Reuse
[0020] batch
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com