Serine protease isolated from the venom of bombus ignitus as fibrinogenolytic and fibrinolytic enzymes
A serine protease, fibrin technology, applied in the field of serine protease, can solve the problem of unclear function of serine protease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Cloning of the gene of the serine protease present in the venom of Bombus rufica
[0027]Total RNA was extracted from the venom glands of Bombus rufica worker bees provided by Dept.of Agricultural Biology in National Academy of Agricultural Science of Rural Development Administration (Dept. of Agricultural Biology in National Academy of Agricultural Science of Rural Development Administration) and extracted using SV Total RNA Isolation System Kit (SV total RNA Isolation System kit) (Promega, USA). Poly(A)+mRNA was then extracted from the total RNA using the PolyATtract mRNA Isolation System kit (Promega, USA). Finally, a cDNA library was constructed using poly(A)+mRNA with Uni-ZAP XP vector and Gigapack III Gold Packing Extract kit (Stratagene, USA), and expressed sequence tags (ESTs) were analyzed. DNA was extracted using Wizard mini-preparation kit (Promega, USA), and its sequence was read using an automatic DNA sequence analyzer (Applied Biosystems, USA)....
Embodiment 2
[0032] Example 2. Venom gland-specific expression, cleavage and O-glycosylation of the serine protease (Bi-VSP) present in the venom of Bombus rufica
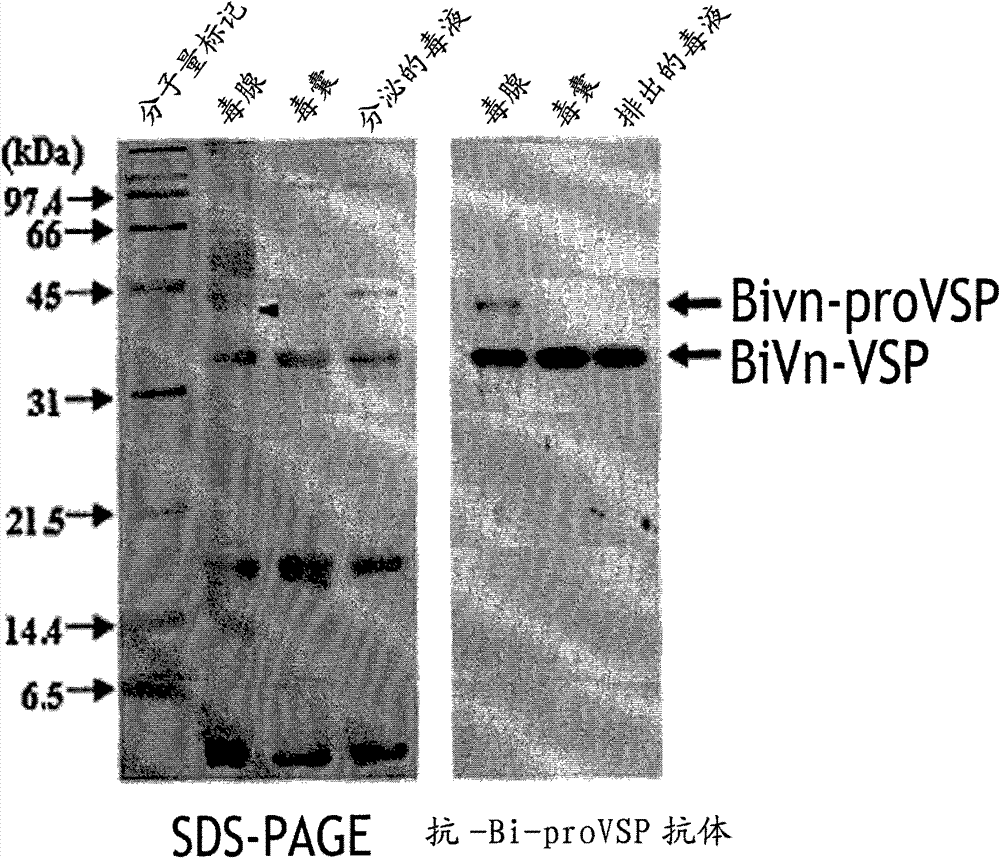
[0033] RNA was extracted from the fat body, midgut, muscle and venom gland of Bombus rufica using the Total RNA Isolation kit (Promega, USA). After adding 5 μg of the extracted RNA to each well, electrophoresis was performed on a 1% formaldehyde agarose gel, and the gel was transferred to a nylon blot membrane (Schleicher & Schuell, Germany), and mixed with [α- 32 P] dCTP (Amersham, USA) labeled cDNA probe hybridization of a serine protease (Bi-VSP) present in the venom of Bombus rubra. As a result, it was found that the mRNA of the serine protease (Bi-VSP) present in the venom of Bombus rufica was specifically present only in the venom gland.
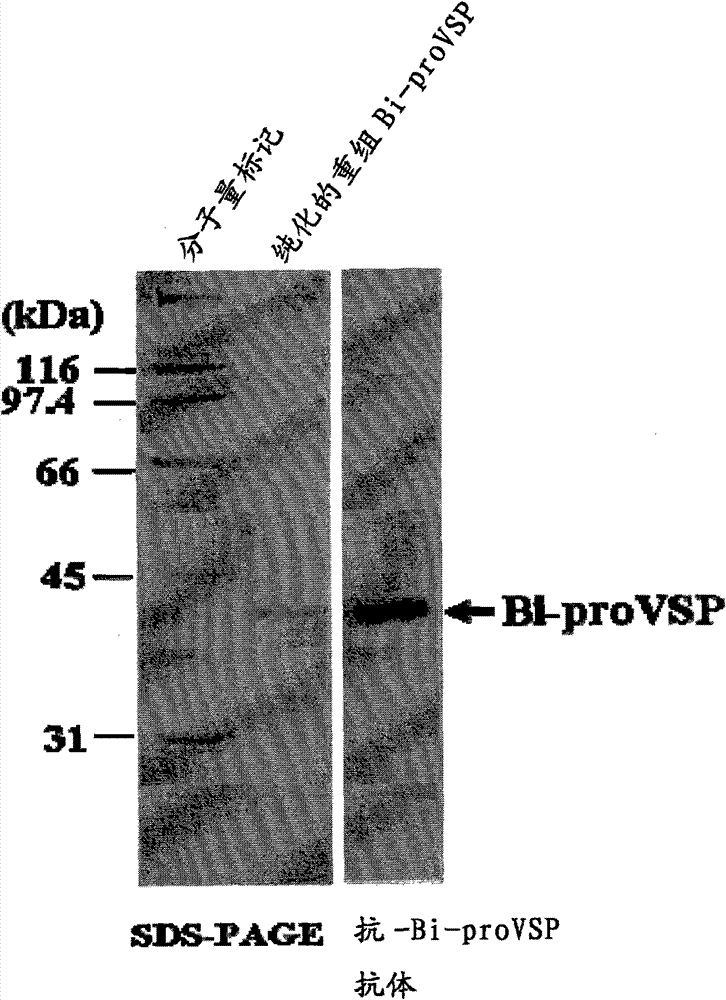
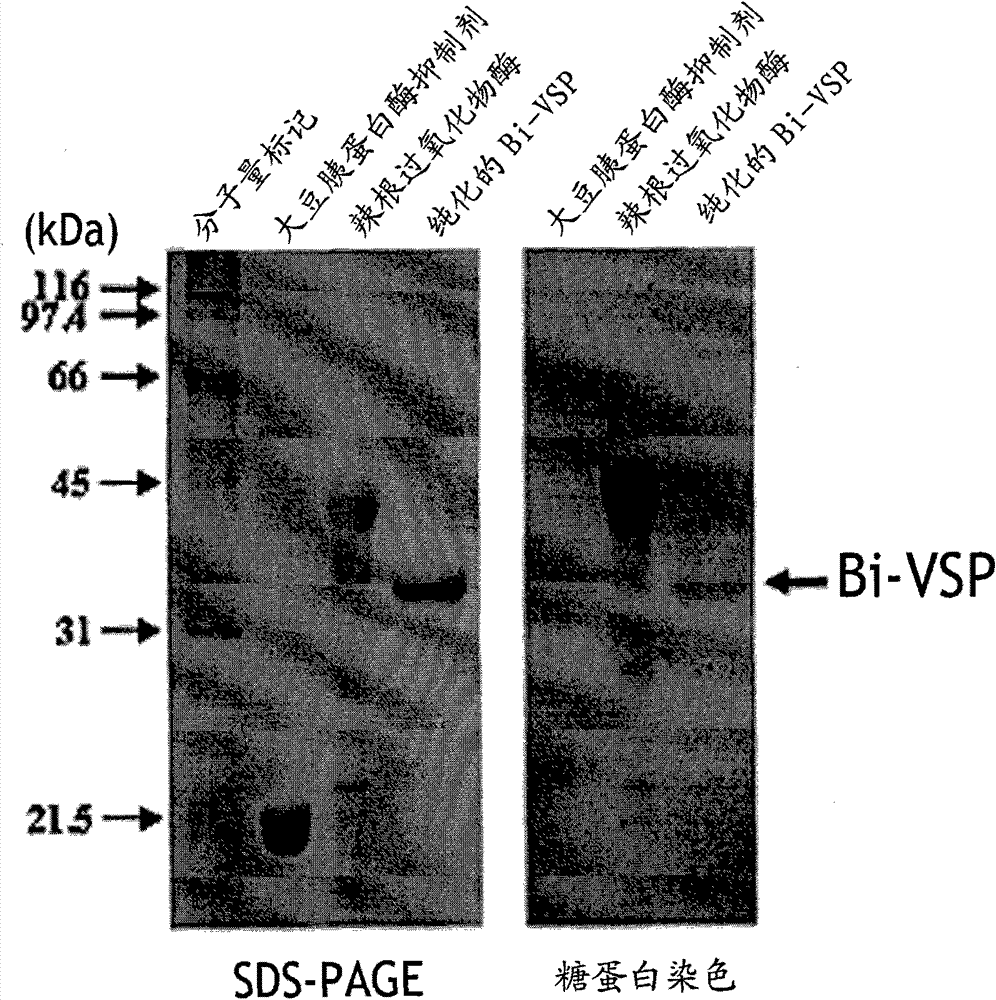
[0034] In order to prepare anti-Bombus venom serine protease (Bi-VSP) antibody, the cDNA of Bombus rufica venom serine protease (Bi-VSP) was inserted into the insect alfalfa inchworm (...
Embodiment 3
[0037] Example 3. Comparison of the amino acid sequences of the serine protease in the venom of Bombus rufica and the serine protease in the snake venom
[0038] The nucleic acid sequences of the serine proteases in the venom of Bombus rubra and the serine proteases in snake venom were aligned using the BLAST program of NCBI (http: / / www.ncbi.nlm.nih.gov / BLAST). When comparing the amino acid sequences of the above two serine proteases, it was found that Bombus vulgaris serine protease (Bi-VSP) and Oscutarin C (GenBank No.AY940204) as a prothrombin activator in the blood coagulation mechanism; Batroxobin (GenBank No.AAA48553) with similar activity; TSV-PA (GenBank No.Q91516) that activates plasmin precursor; PA-BJ (GenBank No.P81824); Halytase (GenBank No.P81176) and RVV-V (GenBank No.P18964) have a certain degree of homology, and the histidine, aspartic acid and serine residues in the serine protease region are highly conserved ( Figure 8 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com