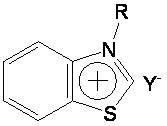
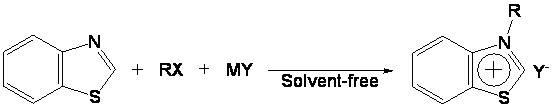
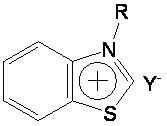
Preparation method and application of novel benzothiazole salt ionic liquid
A technology of benzothiazole salts and ionic liquids, which is applied in the preparation and application fields of new ionic liquids of benzothiazole salts, can solve the problems of small amount of benzothiazole salts, low yield of target products, and normal reaction, etc. Achieve the effects of reducing the use of organic solvents, good economy and environmental protection, and avoiding the use of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: (preparation of ionic liquid)
[0026] Preparation method one of N-n-butyl-benzothiazole tetrafluoroborate ionic liquid
[0027] In a 50 mL single-necked round bottom flask, add 0.05 mol of benzothiazole, 0.05 mol of n-butane bromide, and 0.05 mol of sodium tetrafluoroborate in sequence, and stir and react at 90° C. for 6 h. After the reaction is complete, a yellow paste is obtained. Cool to room temperature, add an appropriate amount of dichloromethane until the solid product is no longer dissolved, filter, and wash the filtrate with distilled water until the washing liquid is tested with silver nitrate and no precipitate is formed. Dichloromethane was recovered under reduced pressure, and the resulting solid was recrystallized in absolute ethanol to obtain a colorless needle-like solid product, which was N-n-butyl-benzothiazole tetrafluoroborate ionic liquid, dried in vacuum, and the calculated yield was 76%, melting point is 92-93°C.
Embodiment 2
[0028] Embodiment 2: (preparation of ionic liquid)
[0029] Preparation method two of N-n-butyl-benzothiazole tetrafluoroborate ionic liquid
[0030] Into a 50 mL single-necked round bottom flask, add 0.05 mol benzothiazole, 0.05 mol n-chlorobutane, 0.05 mol ammonium tetrafluoroborate in sequence, and stir the reaction at 100° C. for 10 h. After completion of the reaction, a light yellow paste was obtained, cooled to room temperature, added an appropriate amount of dichloromethane until the solid product was no longer dissolved, filtered, and the filtrate was washed with distilled water until the washing liquid was tested with silver nitrate and no precipitate was formed. Dichloromethane was recovered under reduced pressure, and the resulting solid was recrystallized in absolute ethanol to obtain a colorless needle-like solid product, which was N-n-butyl-benzothiazole tetrafluoroborate ionic liquid, dried in vacuum, and the calculated yield was 68%, the melting point is 92-93...
Embodiment 3
[0031] Embodiment 3: (preparation of ionic liquid)
[0032] Preparation method three of N-n-butyl-benzothiazole tetrafluoroborate ionic liquid
[0033] Into a 50 mL single-necked round bottom flask, add 0.05 mol of benzothiazole, 0.05 mol of n-butane bromide, and 0.05 mol of potassium tetrafluoroborate in sequence, and stir and react at 90° C. for 7 h. After completion of the reaction, a light yellow paste was obtained, cooled to room temperature, added an appropriate amount of dichloromethane until the solid product was no longer dissolved, filtered, and the filtrate was washed with distilled water until the washing liquid was tested with silver nitrate and no precipitate was formed. Dichloromethane was recovered under reduced pressure, and the resulting solid was recrystallized in absolute ethanol to obtain a colorless needle-like solid product, which was N-n-butyl-benzothiazole tetrafluoroborate ionic liquid, dried in vacuum, and the calculated yield was 81%, melting point...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com