New high-efficiency secretion and expression system of colibacillus and application thereof
A technology of expression cassettes and vectors, applied in the direction of using vectors to introduce foreign genetic material, from leech inhibitors, specific peptides, etc., which can solve problems such as complicated operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
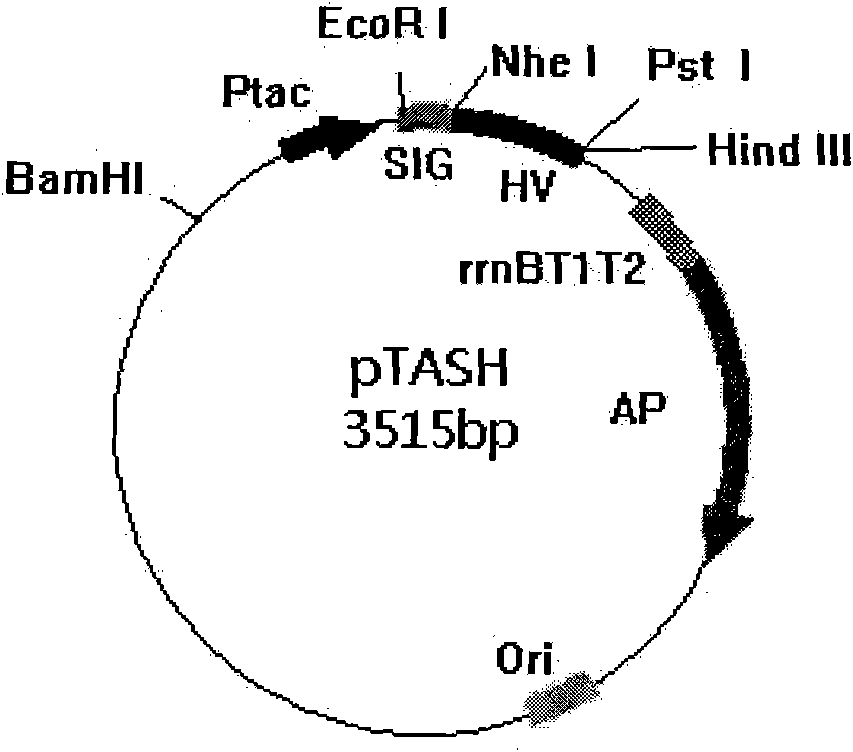
[0023] Amplification of expression cassette gene in embodiment 1 expression vector pTASH
[0024] Extract the template plasmid pTASH from the original Escherichia coli strain containing the pTASH plasmid (Tan Shuhua et al., Chinese invention patent 01113526.3), and design and synthesize the following primers:
[0025] Forward primer pkk223-3TAC-f: 5' GGA TCC AAG CTG TGG TAT GGC TGT GCA GGT CGTAAATC 3' (underline indicates BamH I restriction site)
[0026] Reverse primer pkk223-3rrnBT2-r: 5' GGA TCC AGC GTT TCT GGG TGA GCA AAA ACA GGAAG 3' (underline indicates BamH I restriction site)
[0027] Using the plasmid pTASH as a template, the polymerase chain reaction was carried out under the action of Pfu DNA polymerase. The composition of the 50 μl reaction system is as follows: 20 pmol each of forward primer and reverse primer; 5 units of Pfu DNA polymerase; 5 μl of 10× reaction buffer; 1 μl of dNTP (10 mM each); 2(25mM) 3μl; plasmid DNA 1μl; make up to 50μl with sterile wat...
Embodiment 2
[0029] Example 2 Construction of Double Expression Cassette Recombinant Hirudin Efficient Secretion Expression Plasmid
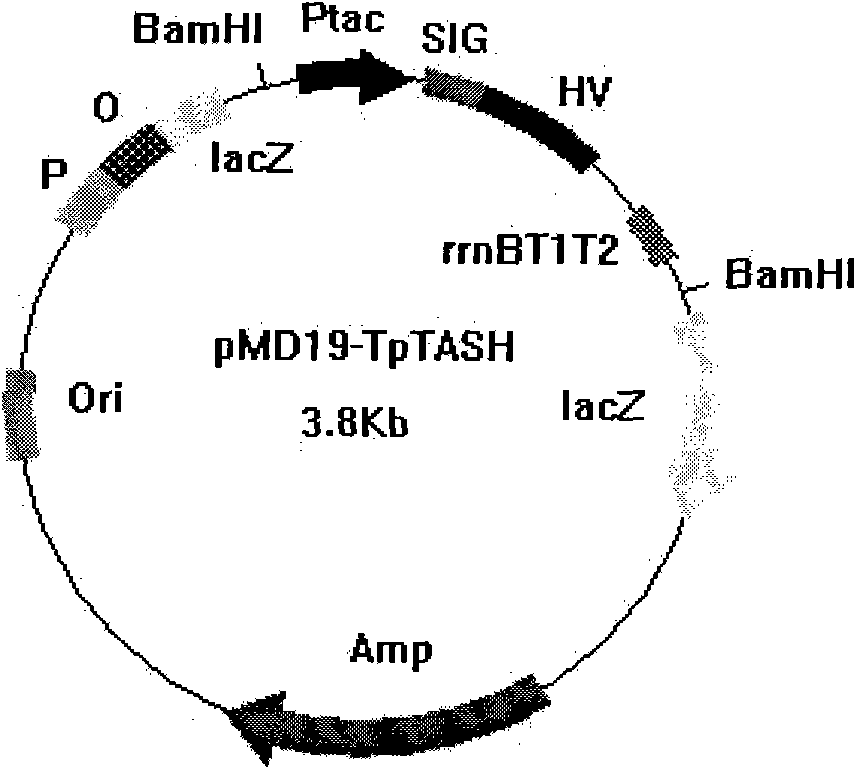
[0030] The plasmid pMD-19T-TASH was extracted by alkaline lysis, digested with BamH I, and DL2,000 TM DNA Marker was used as a control, separated by 1.5% agarose gel electrophoresis, and the digested fragment was recovered to obtain an expression cassette fragment with a size of about 1.1 kb.
[0031] Plasmid pTASH was extracted from the original strain and digested with BamH I to obtain linearized pTASH with the same cohesive ends as the expression cassette fragment. To prevent linearized pTASH from self-cyclization during the ligation reaction, its 5' phosphate group needs to be converted to a hydroxyl group using the dephosphatase CIAP. The 40 μl reaction system is as follows: 4 μl of 10× reaction buffer, 2 μl of CIAP, 34 μl of linearized pTASH, reacted at 37°C for 4 hours; inactivated at 65°C for 30 minutes to terminate the reaction, and then precipitat...
Embodiment 3
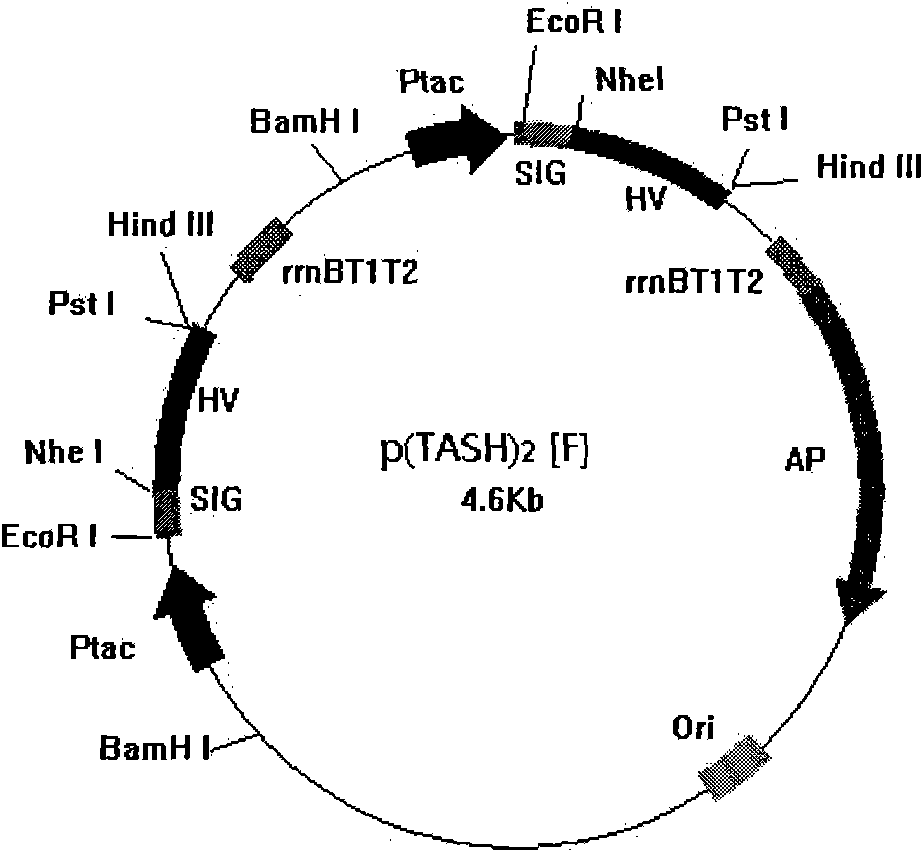
[0035] Example 3 High-efficiency secretory expression of recombinant hirudin III
[0036] The selected expression cassette was inserted into the recombinant hirudin III double expression cassette clone p(TASH) inserted in the forward direction 2 , insert 30mlLBA (tryptone 1%, yeast powder 0.5%, NaCl 1%, Amp100μg / ml, pH7.0) liquid culture medium and carry out seed liquid culture, culture condition is 37 ℃ 220rpm shaking culture 14 hours, then with 2 % inoculum size was transferred to fermentation medium (tryptone 1%, yeast powder 0.5%, sodium glutamate 4%, malt powder 1.0%, Amp100μg / ml, KH 2 PO 4 0.374%, Na 2 HPO 4 12H 2 O 1.75%, pH 7.0), cultured at 37° C. and 220 rpm for 24 hours, and measured the antithrombin activity in the supernatant of the fermentation broth.
[0037] The bioactivity of hirudin was measured with reference to Markwardt's thrombin titration method (F.Markwardt, Hirudin as an inhibitor of thrombin, Methods Enzymol.19 (1970) 924-932.): add 200 μl of 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com