Benzamide analog mediated brain-targeting delivery system
A drug delivery system, a technology of benzamide, applied in the field of pharmaceutical preparations, to avoid risks and complicated drug delivery processes, small molecular weight, and no immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of p-hydroxybenzoic acid-polyethyleneimine-IR820 (p-HA-PEI-IR820)
[0050] 1. Preparation of p-hydroxybenzoic acid-polyethyleneimine (p-HA-PEI)
[0051] Weigh 0.130g of PEI and dissolve in 3ml of DMF, dissolve 7.5mg of p-hydroxybenzoic acid and 9.5mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride in 1ml of DMF, and stir It was added dropwise into the DMF solution of PEI, and stirred overnight at room temperature to obtain a white suspension. Discard the precipitate after centrifugation, add 100ml of cold ether to the supernatant for precipitation treatment, discard the supernatant after centrifugation, and wash the precipitate with cold ether 3 times, 10ml each time. After vacuum drying for 24 hours, the product was dissolved in a small amount of double-distilled water (dH2O), eluted with dH2O on a G-25 gel column, and the corresponding components were collected and freeze-dried to obtain p-HA-PEI. The results of proton nuclear magnetic spe...
Embodiment 2
[0059] Animal Test of p-Hydroxybenzoic Acid-Polyethyleneimine-IR820 (p-HA-PEI-IR820) Intracerebral Drug Delivery System
[0060] 1. Distribution of p-hydroxybenzoic acid-polyethyleneimine-IR820 mouse living tissue
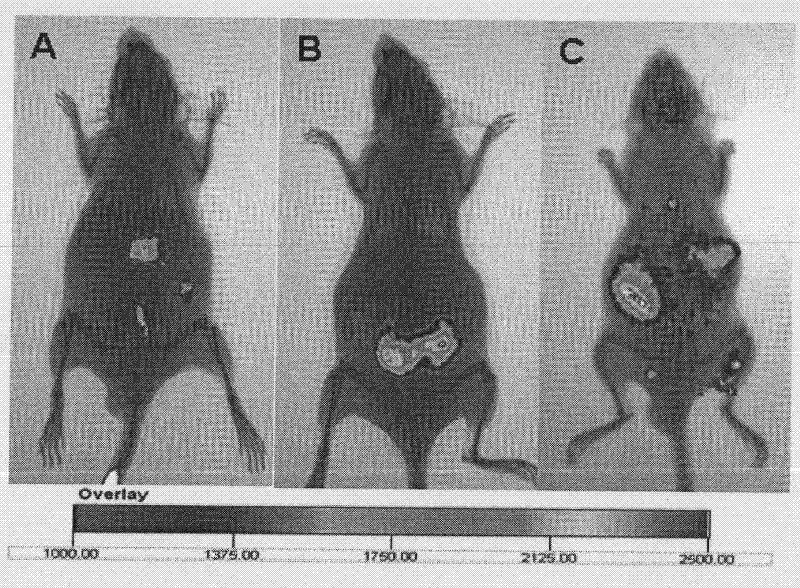
[0061] Weigh the appropriate amount of p-HA-PEI-IR820, PEI-IR820 and IR820 respectively, dissolve them with normal saline to prepare a 1 mg / ml solution, and inject 100 μl / mouse into the tail vein, respectively, at 0.5, 2, 4, The mice were anesthetized with 10% chloral hydrate at 12 and 24 hours, and the fluorescence distribution in the mice was observed in the live animal imaging system. It can be seen from the distribution diagram that p-HA-PEI-IR820 has obvious fluorescence distribution in the mouse brain, while IR820 and PEI-IR820 have no fluorescence distribution in the brain, suggesting that p-HA-PEI-IR820 can pass through p-hydroxybenzene Formic acid mediates penetration of the blood-brain barrier into the brain ( figure 1 ).
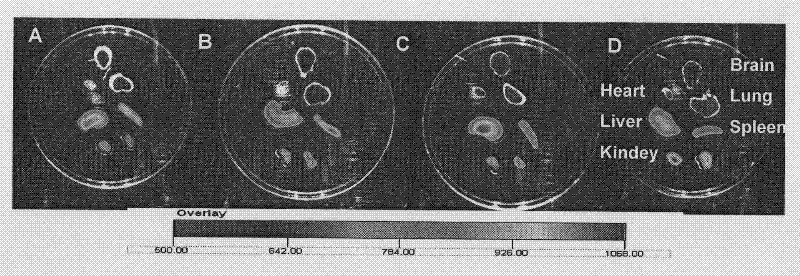
[0062] 2. Distribution of p-...
Embodiment 3
[0067] p-Hydroxybenzoic acid-polyethyleneimine 125 In vivo distribution test of marker I in mice
[0068] 1. p-hydroxybenzoic acid-polyethyleneimine 125 I mark ( 125 I-p-HA-PEI)
[0069] p-HA-PEI (65μg / μl) 50μl, add Na 125 I 0.702mCi, 40°C water bath reaction for 5 minutes, G-25 gel column purification, HPLC gradient elution, C-18 chromatographic column detection, labeling rate 100%, product activity 0.636mCi.
[0070] 2, 125 Distribution test of I-labeled p-hydroxybenzoic acid-polyethyleneimine in mice
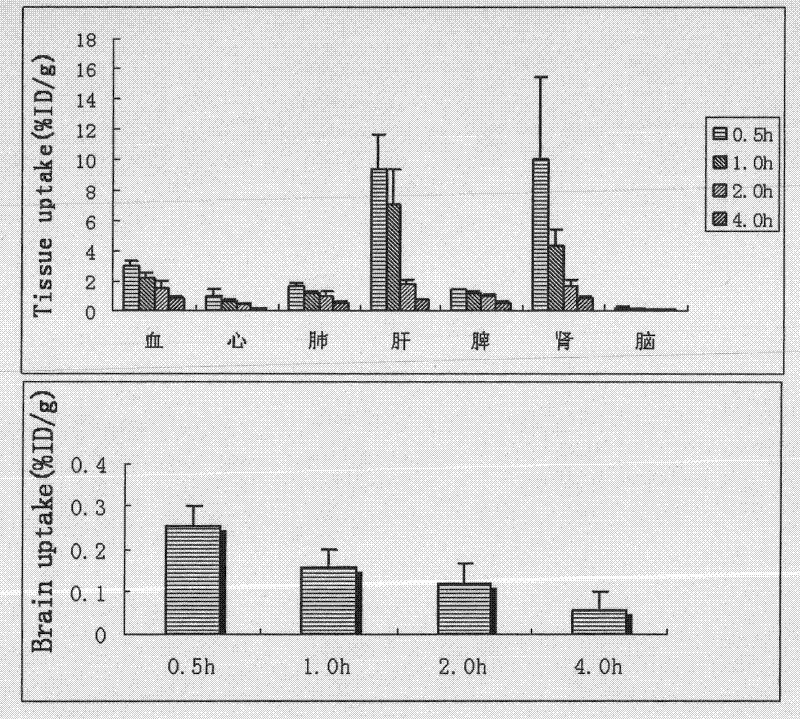
[0071] mouse tail vein injection 125 I-p-HA-PEI solution 100 μl / only (0.199 μCi / μl, 1.0 μg / μL), anesthetized mice with 10% chloral hydrate at 0.5, 1, 2 and 4 hours after administration respectively, and cardiac perfusion normal saline 100ml / Only organs or tissues such as heart, lung, liver, spleen, kidney and brain were taken, weighed, radioactive counts were determined, and the percentage of tissue per unit weight in the total injected radioactive counts (ID% / g) was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com