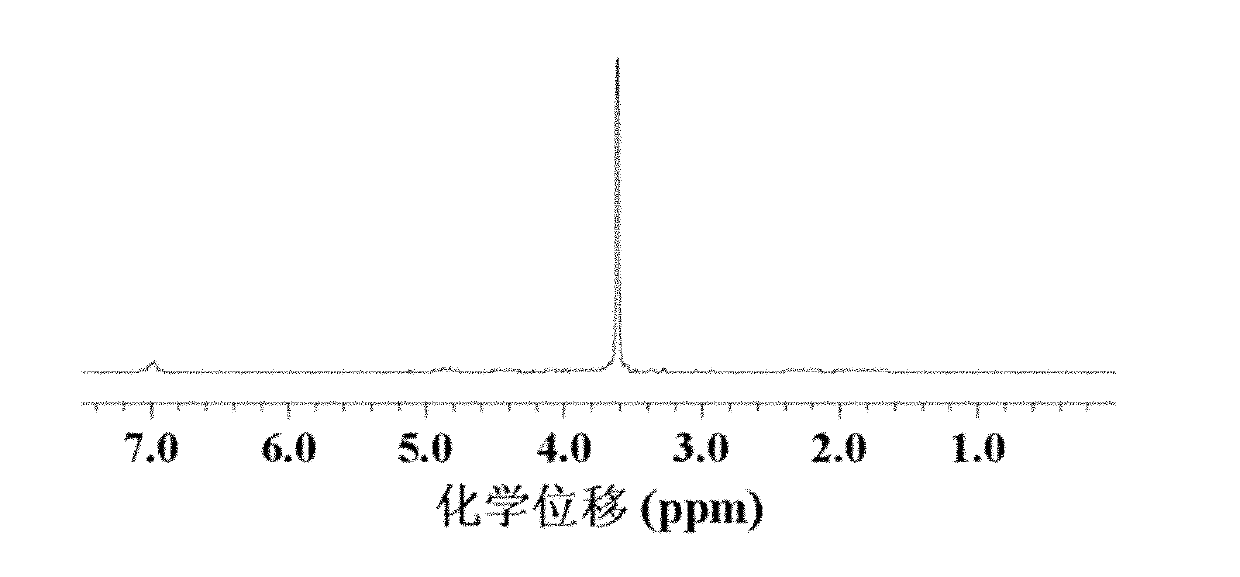
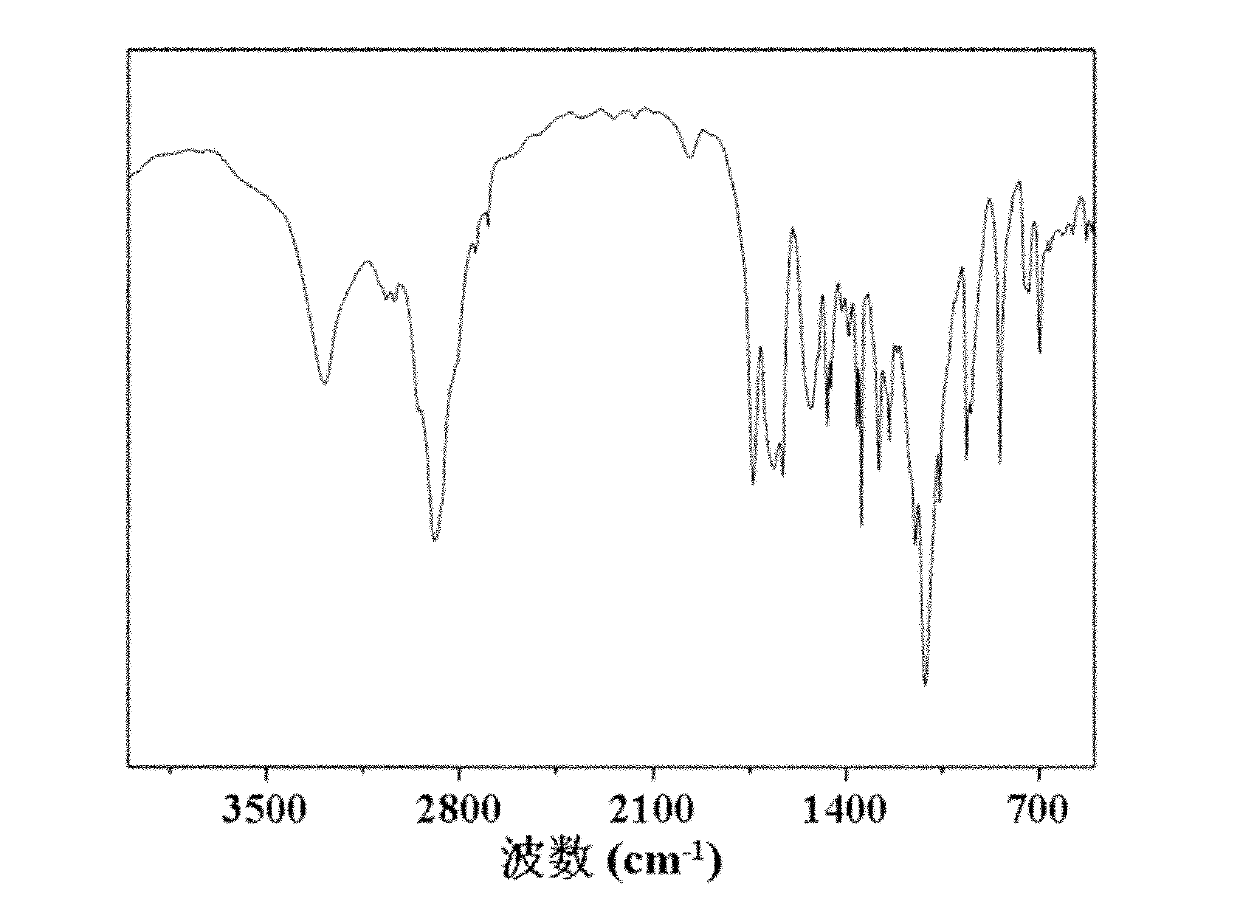
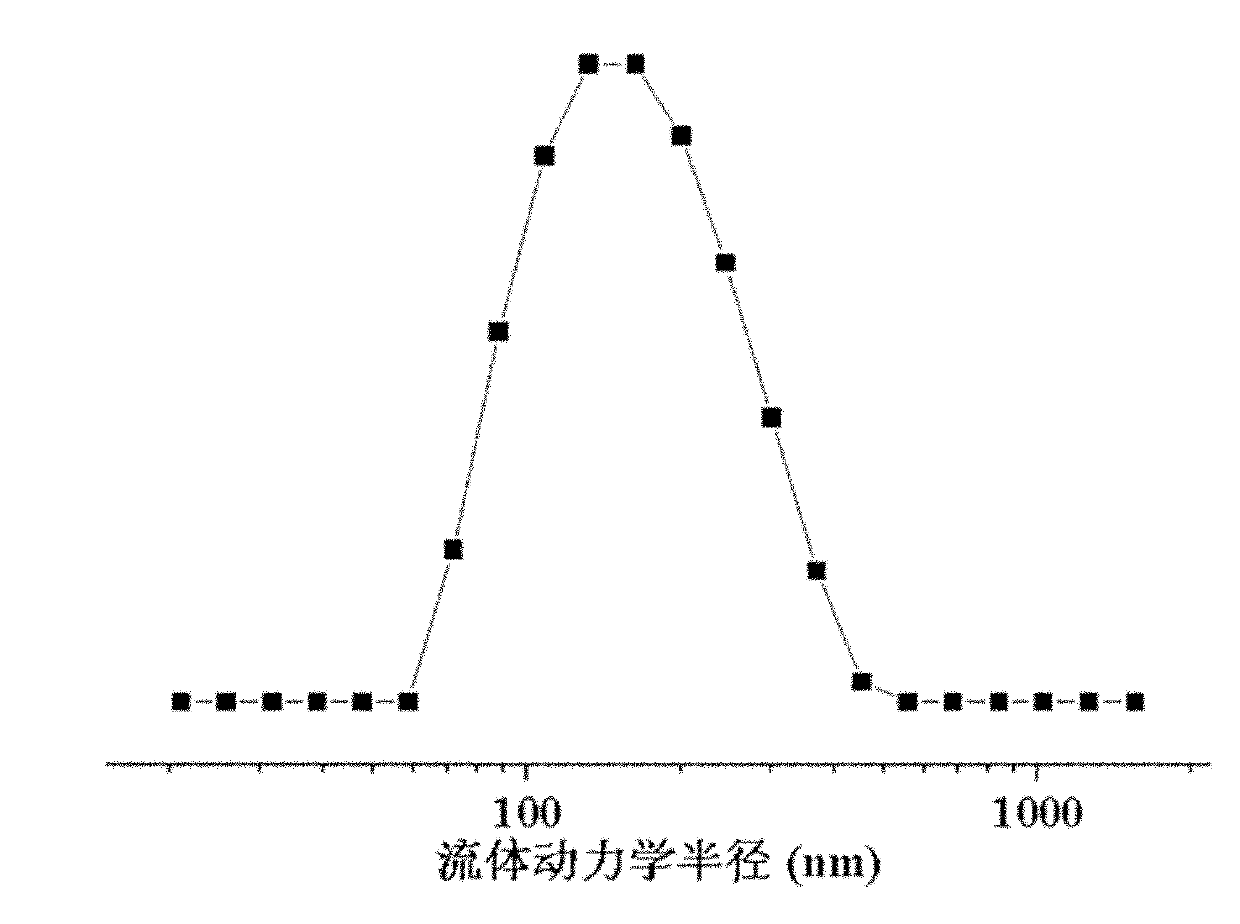
Preparation method of polyamino acid and polyamino acid nano-hydrogel
A technology of polyamino acids and amino acids, applied in the field of polyamino acids, can solve problems such as application limitations, unfavorable large-scale production, and complicated hydrogel preparation process, and achieve the effect of simple steps and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides a kind of preparation method of polyamino acid, comprises the following steps:
[0029] The terminal aminated hydrophilic polymer, L-cystine-N-internal carboxylic acid anhydride and amino acid-N-internal carboxylic anhydride are dissolved in an organic solvent, and the polyamino acid is obtained after stirring and reacting.
[0030] The present invention uses end-aminated hydrophilic polymers, L-cystine-N-internal carboxylic acid anhydride and amino acid-N-inner carboxylic anhydride as raw materials to prepare polyamino acids capable of forming nano-hydrogels. Under the initiation of hydrophilic polymer, L-cystine-N-internal carboxylic acid anhydride and amino acid-N-internal carboxylic acid anhydride undergo ring-opening polymerization reaction; wherein, L-cystine-N-internal carboxylic acid anhydride contains two A ring can form a cross-linked structure during ring-opening polymerization, thereby obtaining a polyamino acid that can form a...
Embodiment 11
[0090] The preparation of embodiment 11 gamma-benzyl-L-glutamate-N-internal carboxylic acid anhydride
[0091] Mix 1 mol of L-glutamic acid and 3 mol of benzyl alcohol at 0°C, add 1.5 mol of concentrated sulfuric acid dropwise under the stirring condition of a stirrer, after the dropwise addition, raise the temperature to 70°C for 6 hours, after the reaction, add 3 mol of carbonic acid The sodium hydrogen solution neutralizes the reaction mixture, and after filtration, washing, recrystallization and freeze-drying, γ-benzyl-L-glutamic acid ester is obtained.
[0092] Mix 1 mol of the γ-benzyl-L-glutamate with 0.6 mol of bis(trichloromethyl)carbonate at 25°C, add tetrahydrofuran, heat to 50°C for 2 hours, and after the reaction, put The reaction mixture was settled in excess petroleum ether, separated, washed, recrystallized and dried to obtain γ-benzyl-L-glutamic acid ester-N-internal carboxylic acid anhydride.
Embodiment 12
[0093] The preparation of embodiment 12L-phenylalanine-N-inner carboxylic acid anhydride
[0094] Mix 1 mol of L-phenylalanine and 0.6 mol of bis(trichloromethyl)carbonate at 25°C, add tetrahydrofuran, heat to 50°C for 2 hours, after the reaction, settle the reaction mixture in excess petroleum ether, After separation, washing, recrystallization and drying, L-phenylalanine-N-internal carboxylic acid anhydride is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com