Absorbing agent for separating formaldehyde from air and preparation method thereof
An adsorbent and formaldehyde technology, which is applied in the field of adsorbents for efficient separation of formaldehyde and its preparation, can solve the problems of increased difficulty in maintenance and management, short service life, and low adsorption capacity, and achieve clean production, long service life, and adsorption The effect of large capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
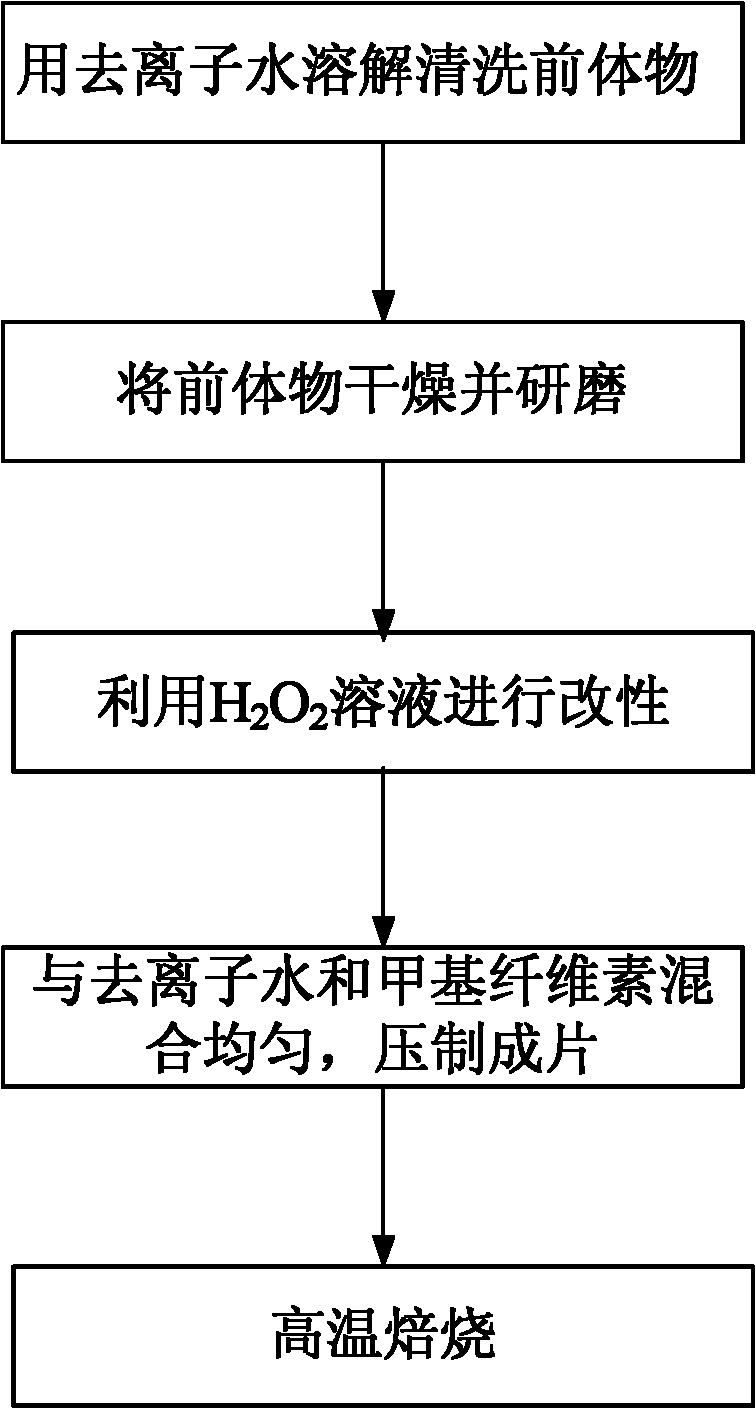
[0024] The present invention also provides a method for preparing an adsorbent for separating formaldehyde from air, such as figure 1 As shown, it specifically includes the following steps:
[0025] Step 1: Dissolve and clean the precursor directly with deionized water. The precursor is the red mud produced by the Bayer aluminum smelting process, and its components are silica, iron oxide, alumina, calcium oxide, sodium oxide, and antimony oxide As well as the ignition loss part, the mass ratio of the precursor to the deionized water is 1:20 to 1:10;
[0026] Step 2: Take out the cleaned precursor, place it in a drying oven, and dry it at 100-110° C. for 12-16 hours. Grinding the dried precursor to a particle size of 0.18-0.28 mm;
[0027] Step 3: Add H with a concentration ranging from 1% to 30% 2 o 2 The solution and the 0.18-0.28mm precursor are mixed according to the liquid-solid ratio of 1:5-1:10, and chemically modified on a rotary evaporator. The modification soaking...
Embodiment 1
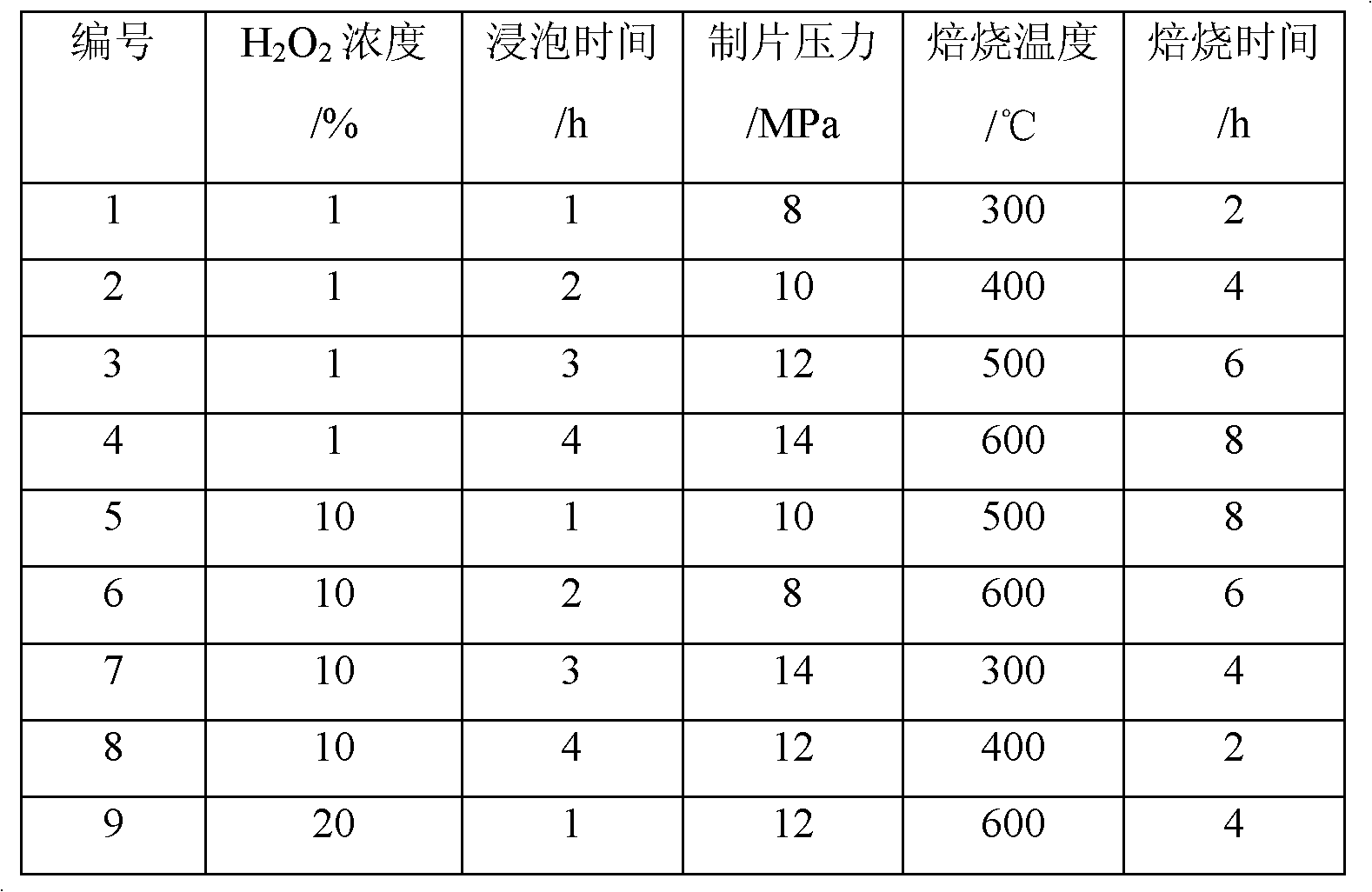
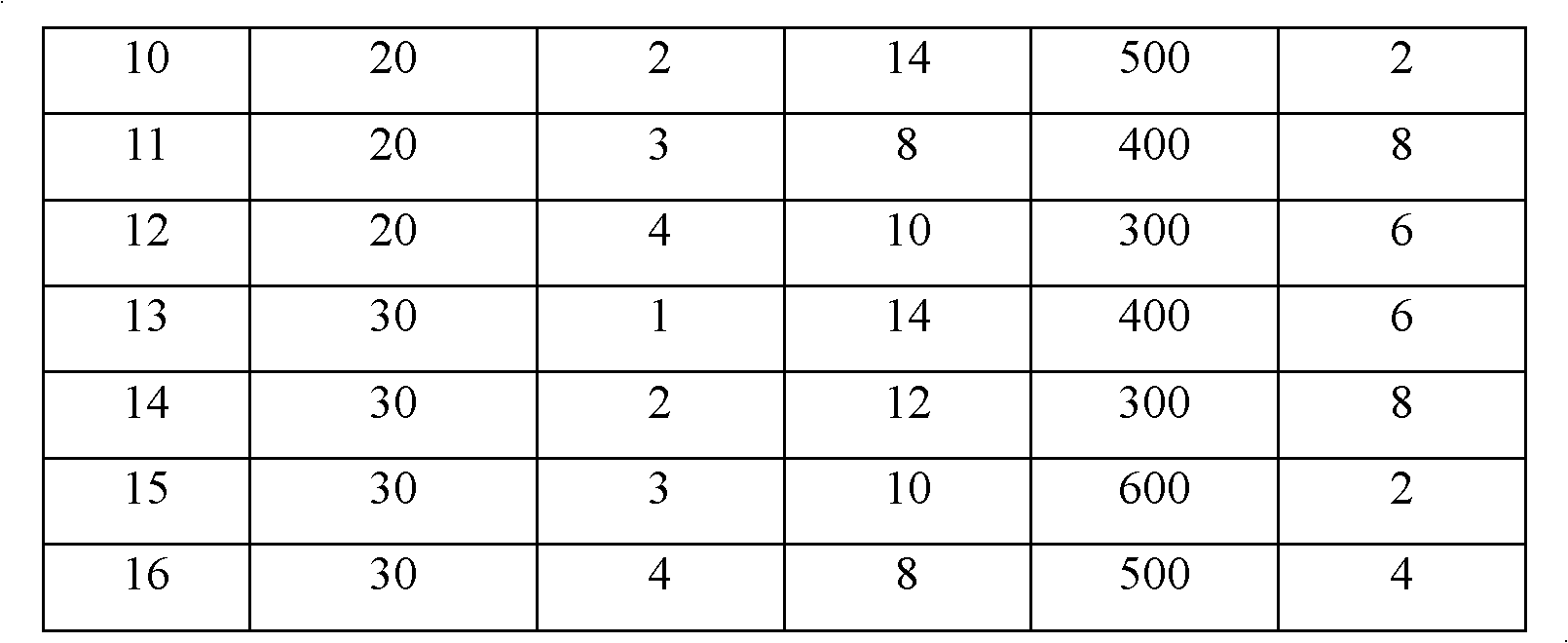
[0032] Example 1 : The preparation number is 1~16# adsorbent, the preparation method is as follows:
[0033] Step 1: washing the precursor with deionized water, the mass ratio of the precursor to deionized water is 1:20;
[0034] Step 2: Take out the cleaned precursor, place it in a drying oven, dry it at 110° C. for 12 hours, and grind the obtained powdery precursor to a particle size of 0.18 mm;
[0035] Step 3: 100ml of different concentrations of H 2 o 2 The solution was mixed with 20g of dry and ground precursor, and modified on a rotary evaporator after mixing. 2 o 2 The concentration of the solution is shown in Table 1 respectively to obtain the modified precursor;
[0036] Step 4: Mix the modified precursor with deionized water and methylcellulose according to the weight ratio of 100:27:2.7, and press it into a tablet under different tableting pressures (as shown in Table 1) , to obtain a sheet-shaped modified precursor;
[0037] Step 5: Place the obtained flak...
Embodiment 2
[0045] Example 2 : Preparation number is 17~21# adsorbent
[0046] Step 1: washing the precursor with deionized water, the mass ratio of the precursor to deionized water is 1:20;
[0047] Step 2: Take out the cleaned precursor, place it in a drying oven, dry it at 110° C. for 12 hours, and grind the obtained powdery precursor to a particle size of 0.18 mm;
[0048] Step 3: Add 100ml H 2 o 2 The solution was mixed with 20 g of dry and ground precursors, and then modified on a rotary evaporator with a rotational speed of 60 r / min, a water bath temperature of 50°C, soaking time and H 2 o 2 The concentrations of the solutions are shown in Table 3 respectively;
[0049] Step 4: Mix the modified precursor with deionized water and methyl cellulose evenly according to the weight ratio of 100:27:2.7, and press it into a tablet under different tableting pressures (as shown in Table 3) , to obtain a sheet-shaped modified precursor;
[0050] Step 5: Place the obtained flake-shaped...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com