Hypha mycin gene and karyogamy gene-engineering bacteria thereof
A kind of pectin and gene technology, applied in genetic engineering, plant genetic improvement, bacteria and other directions, can solve the problems of no E. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The construction of embodiment 1 plectasin prokaryotic fusion genetic engineering bacteria
[0033] 1.1 Design of Plectasin gene based on Escherichia coli preferred codons
[0034] According to the preference of Escherichia coli (Escherichia coli) codon usage (http: / / www.kazusa.or.jp / codon / ) optimization design, the obtained plectasin gene has as shown in the sequence table SEQ ID NO.1 Nucleotide sequence.
[0035] 1.2 Construction of plectasin prokaryotic fusion expression vector
[0036] Design the recognition cleavage site (IEGR) coding sequence of factor Xa at the 5′-end of the Plectasin gene, which is used to cut the fusion protein to obtain recombinant Plectasin, and design the multiple cloning sites of the vector that are not available in the Plectasin gene at both ends of the gene The restriction endonuclease BamHI and XhoI restriction sites on the site were directly synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd., and the plectasin ...
Embodiment 2
[0039] Induced expression of embodiment 2 fusion protein and purification of recombinant plectasin
[0040] 2.1 Induced expression of fusion protein
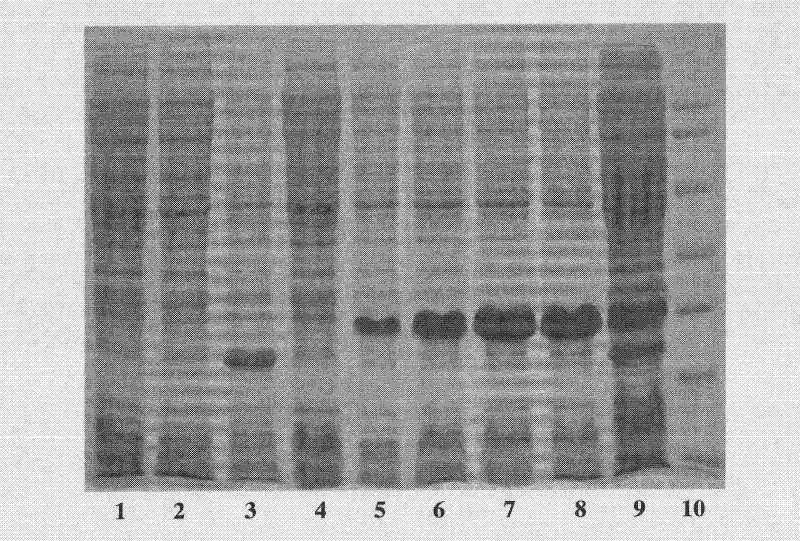
[0041] Inoculate the transformed colonies in 10 mL of LB medium containing 100 μg / mL Amp, culture overnight in a shaker at 37°C, transfer 1% of the inoculum to 100 μg / mL Amp in TB medium, and wait for the bacteria to grow to logarithmic In the growth phase, when OD600 is about 1.0, add 100mmol / LIPTG to make the final concentration 0.4mmol / L. After induction at 30°C for different times, centrifuge at 5000rpm for 5min, wash once with PBS buffer (pH 7.4), collect the cells by centrifugation, and SDS- PAGE electrophoresis showed ( figure 1), the molecular weight of the protein expressed in the bacterial protein was close to the expected fusion protein molecular weight (22.4 kDa), and the expression level of the fusion protein Trx-plectasin reached the highest level at 4 h of induction, accounting for 53.6% of the total cell protein...
Embodiment 3
[0044] The activity of embodiment 3 recombinant Plectasin
[0045] 3.1 Hemolytic property of recombinant plectasin
[0046] Use a 10mL vacuum blood collection tube (containing heparin sodium as an anticoagulant) to collect blood from the ear vein of Japanese long-eared white rabbits, wash the cells three times with 10mM phosphate buffer (PBS, pH 7.3), and then centrifuge at 4°C and 1500rpm 10min until the supernatant is colorless and transparent. Take 180 μL of 10 mM PBS suspension of red blood cells (concentration of red blood cells is about 2%), mix with 20 μL of purified recombinant plectasiacin diluted in different ratios, and the mixed system is incubated at 37° C. for 30 minutes, and then centrifuged at 1500 rpm for 5 minutes. The supernatant was transferred to a 96-well plate, and the absorbance was measured at 540 nm using a microplate reader. The values of hemolysis percentage 0% and 100% were determined simultaneously under the same conditions using 10 mM PBS and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com