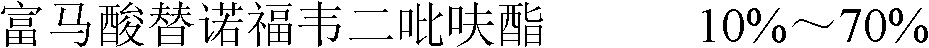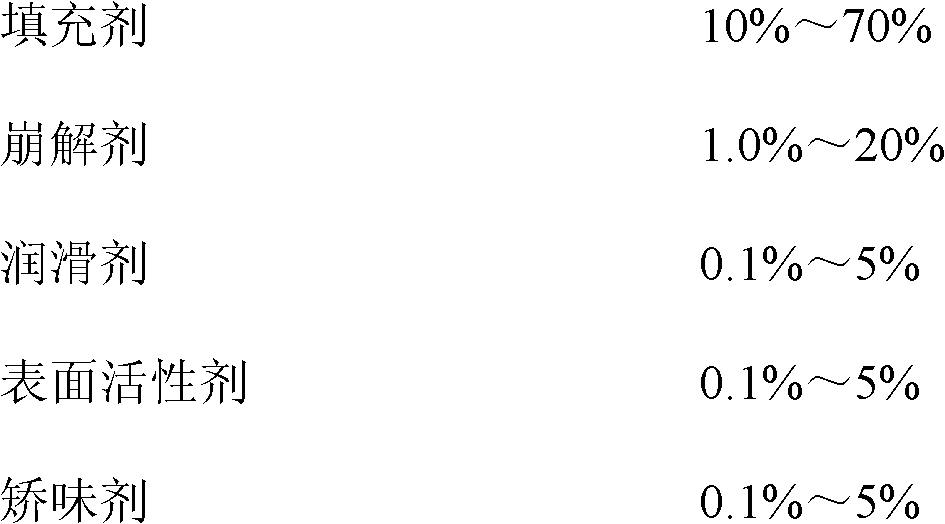Tenofovir disoproxil fumarate dispersible tablets and preparation method thereof
A technology for tenofovir fumarate and disoproxil, which is applied in the field of tenofovir disoproxil fumarate dispersible tablets and their preparation, can solve problems such as restriction and poor compliance, and achieve fast dissolution rate , Small weight difference, one-sided smooth effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] prescription:
[0064]
[0065] Preparation method: fully mix tenofovir disoproxil fumarate, mannitol, microcrystalline cellulose, lactose, low-substituted hydroxypropyl cellulose, and aspartame, and pulverize them through a 100-mesh sieve. 5% starch aqueous solution is made into 20-mesh wet granules, dried at 55°C for 3 hours, sieved with 18-mesh sieve, added with crospovidone, sodium lauryl sulfate, and magnesium stearate and mixed thoroughly, then the content is determined , the adjustment sheet is repressed into a sheet.
Embodiment 2
[0067] prescription:
[0068] Preparation method: fully mix tenofovir disoproxil fumarate, mannitol, microcrystalline cellulose, lactose, low-substituted hydroxypropyl cellulose, and aspartame, and pulverize them through a 100-mesh sieve. 3% povidone aqueous solution is made into 20-mesh wet granules, dried at 55°C for 3.5 hours, sieved with 18-mesh sieves, added crospovidone, sodium lauryl sulfate, micropowder silica gel, magnesium stearate and fully mixed After uniformity, measure the content, adjust the weight of the tablet and press it into a tablet.
Embodiment 3
[0070] prescription:
[0071] Preparation method: fully mix tenofovir disoproxil fumarate, microcrystalline cellulose, lactose, low-substituted hydroxypropyl cellulose, and aspartame, and pulverize them through a 100-mesh sieve, add an appropriate amount of 5% starch The aqueous solution is made into 20-mesh wet granules, dried at 50°C for 3 hours, sieved with 18-mesh sieve, added with crospovidone, sodium lauryl sulfate, and magnesium stearate and mixed thoroughly, then measuring the content and adjusting the tablet Pressed into pieces.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com