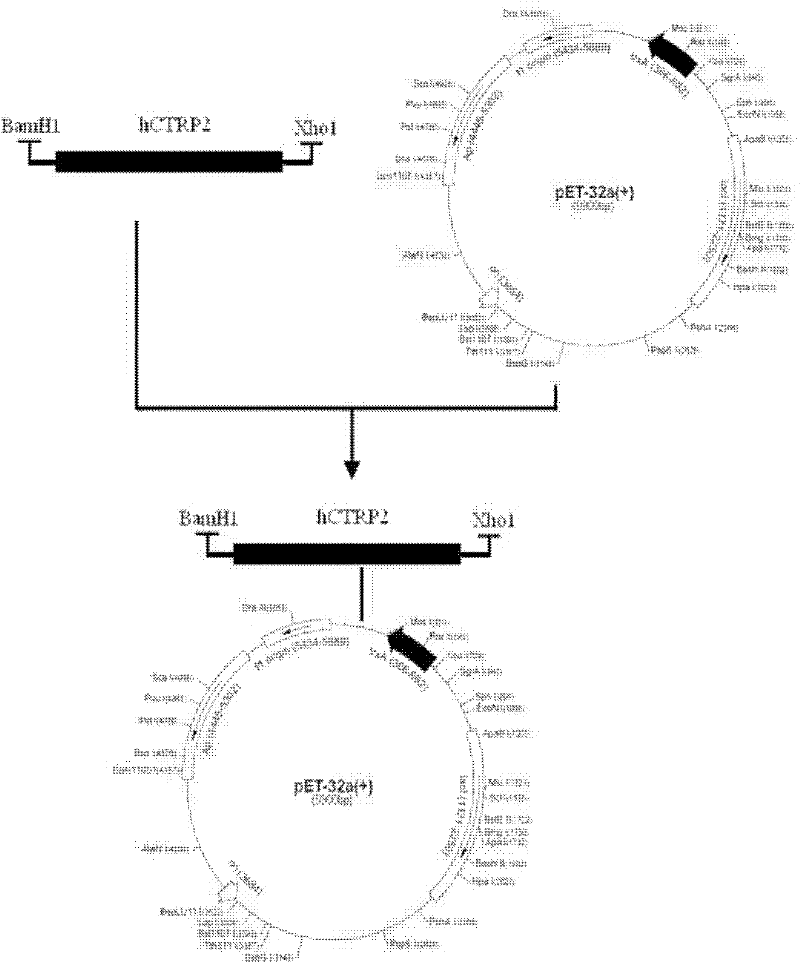
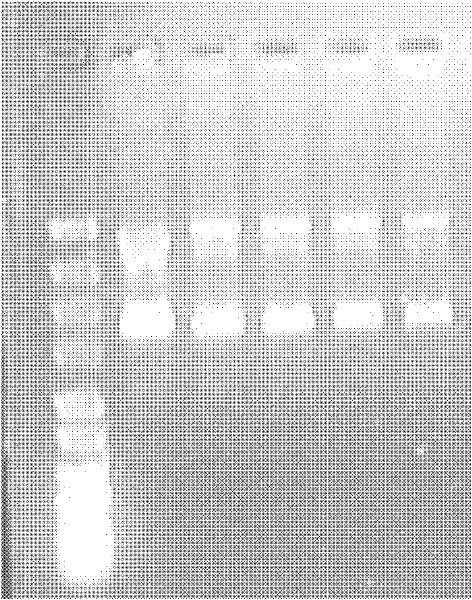
Efficient method for producing Trx-hCTRP2
A high-efficiency, pmd20-t technology, applied in the field of high-efficiency production of Trx-hCTRP2, can solve the problems of inactivation of the target product, instability of hCTRP2 in yeast, inability to obtain full-length and uniform hCTRP2 protein, etc., and achieve the effect of good biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] In the present invention, the Escherichia coli expression strain BL21 (DE3), the vector amplification strain TOP10 and the expression vector pET32-a (+) were all purchased from Invritrogen Company of the United States.
[0029] The medium formula used is as follows:
[0030] 1) LB liquid medium: NaCl 10g, peptone 10g, yeast extract 5g, distilled water 1L, autoclave, store at room temperature;
[0031] 2) LB / Amp plate: NaCl 10g, peptone 10g, yeast extract 5g, distilled water 1L, agar powder 15g, after autoclaving, cool to below 70°C, add 1mL Ampicillin (100mg / ml), mix well and pour the plate , stored in the dark at 4°C;
[0032] 3) LB / Amp medium: NaCl 10g, peptone 10g, yeast extract 5g, distilled water 1L, after autoclaving, cool to below 70°C, add 1mL Ampicillin (100mg / ml), mix well, store at 4°C. LB liquid medium: NaCl 10g, peptone 10g, yeast extract 5g, distilled water 1L, autoclave, store at room temperature;
[0033] 4) 50×TAE agarose gel electrophoresis buffer: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com