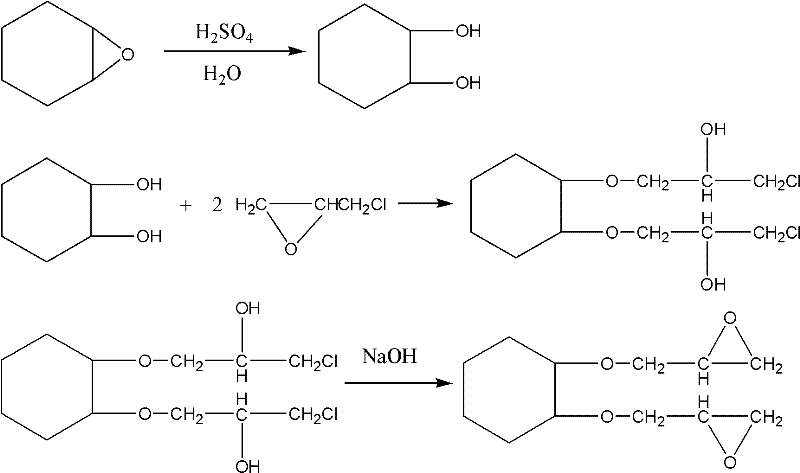
Preparation process of 1,2-cyclohexanediol diglycidyl ether
A technology of diglycidyl ether and cyclohexanediol, applied in organic chemistry and other directions, can solve the problem of high energy consumption and achieve the effects of low chromaticity, environmental protection comprehensive cost and low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 540 kg of water into the reaction kettle, add 10 kg of sulfuric acid as a catalyst, add 980 kg of epoxycyclohexane dropwise at 50°C, complete the dropwise addition within 1 hour, and hydrolyze for 2 hours to obtain cyclohexanediol The aqueous solution was transferred to the etherification tank by siphon, and 720 kg of solvent toluene was added, and azeotropic dehydration was carried out at 95°C for 6 hours. Hexylene glycol / toluene solution, boron trifluoride etherate complex was added thereto, 190 kg of epichlorohydrin was added dropwise at 55°C, the addition was completed within 2 hours, and the obtained intermediate solution was transferred to Wash the kettle with water, add 280 kg of sodium hydroxide solution with a mass percentage concentration of 30% dropwise, drop it at 50°C for 2 hours, react for 2 hours, let it stand to remove the lower layer of saline solution, add 200 kg of water, mix it well and then let it stand to remove The lower aqueous solution was r...
Embodiment 2
[0020] Add 540 kg of water into the reaction kettle, add 10 kg of sulfuric acid as a catalyst, add 980 kg of epoxycyclohexane dropwise at 55°C, complete the dropwise addition within 1.5 hours, and hydrolyze for 2 hours to obtain cyclohexanediol The aqueous solution was transferred to the etherification kettle by siphon, and 720 kg of solvent benzene was added, and azeotropic dehydration was carried out at 76°C for 7 hours. Hexylene glycol / benzene solution, adding boron trifluoride etherate complex therein, dripping 190 kilograms of epichlorohydrin at 60 ℃, adding dropwise within 1.5 hours, and transferring the intermediate solution obtained after reacting for 4 hours to Wash the kettle with water, add 280 kg of sodium hydroxide solution with a mass percentage concentration of 30% dropwise, drop it at 50°C for 2 hours, react for 2 hours, let it stand to remove the lower layer of saline solution, add 200 kg of water, mix it well and then let it stand to remove The lower aqueous ...
Embodiment 3
[0022] Add 540 kg of water into the reaction kettle, add 10 kg of sulfonic acid-based polystyrene gel-type acidic ion exchange resin as a catalyst, add 980 kg of epoxycyclohexane dropwise at 50°C, and complete the dropwise addition within 1 hour , hydrolysis reaction for 2 hours, the prepared cyclohexanediol aqueous solution was transferred to the etherification kettle by siphon, 720 kg of solvent xylene was added, and azeotropic dehydration was carried out at 120°C for 4 hours, the condensate was separated from the water by the oil-water separator, and the solvent was refluxed to the etherification kettle until no water is taken out to obtain a cyclohexanediol / xylene solution, to which boron trifluoride etherate complex is added, and 190 kg of epichlorohydrin is added dropwise at 50°C, within 1 hour After the addition is completed, the obtained intermediate solution is transferred to a washing kettle after 4 hours of reaction, and 280 kilograms of potassium hydroxide solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com