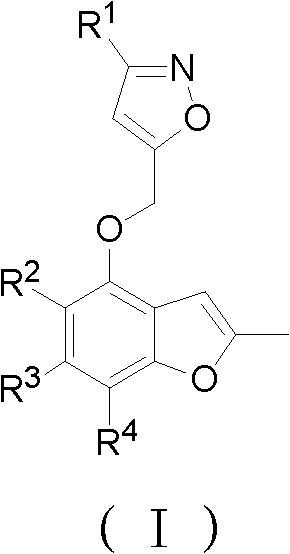
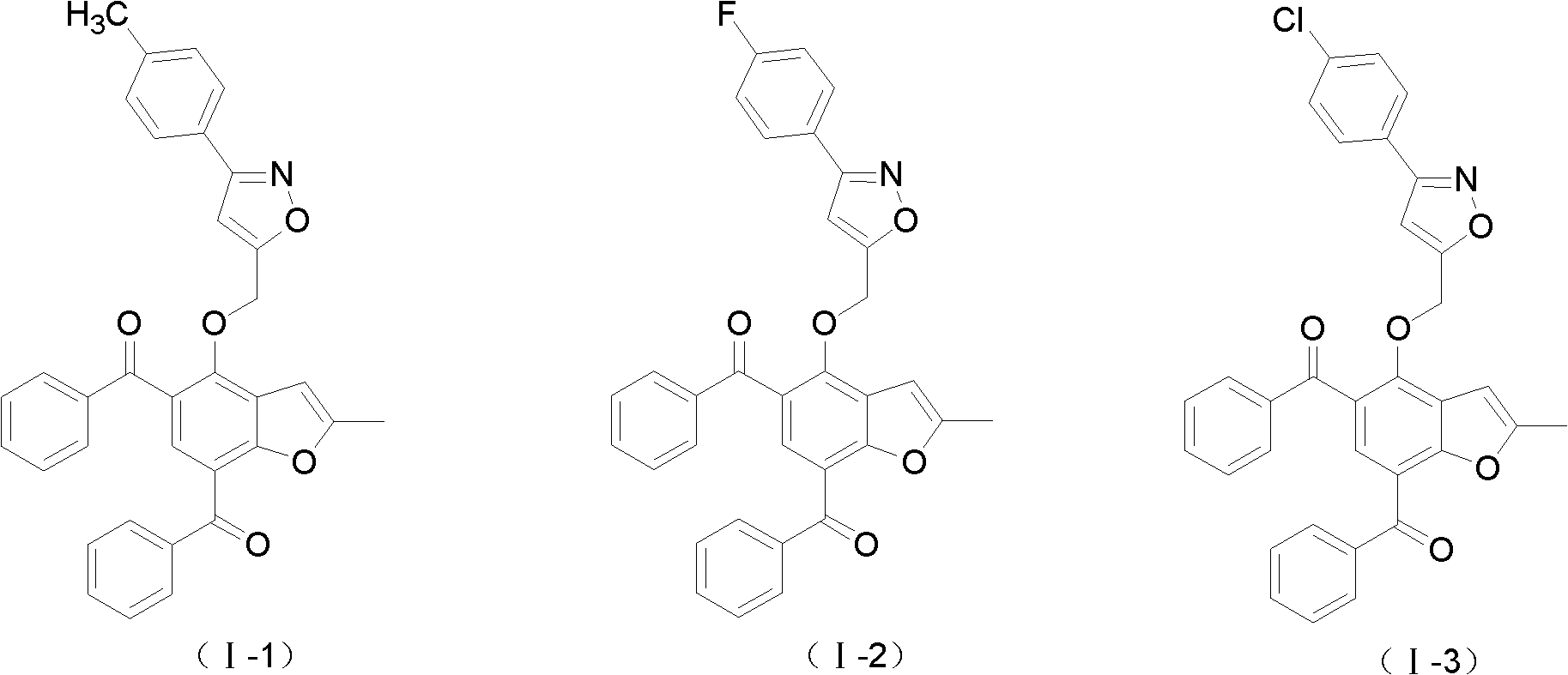
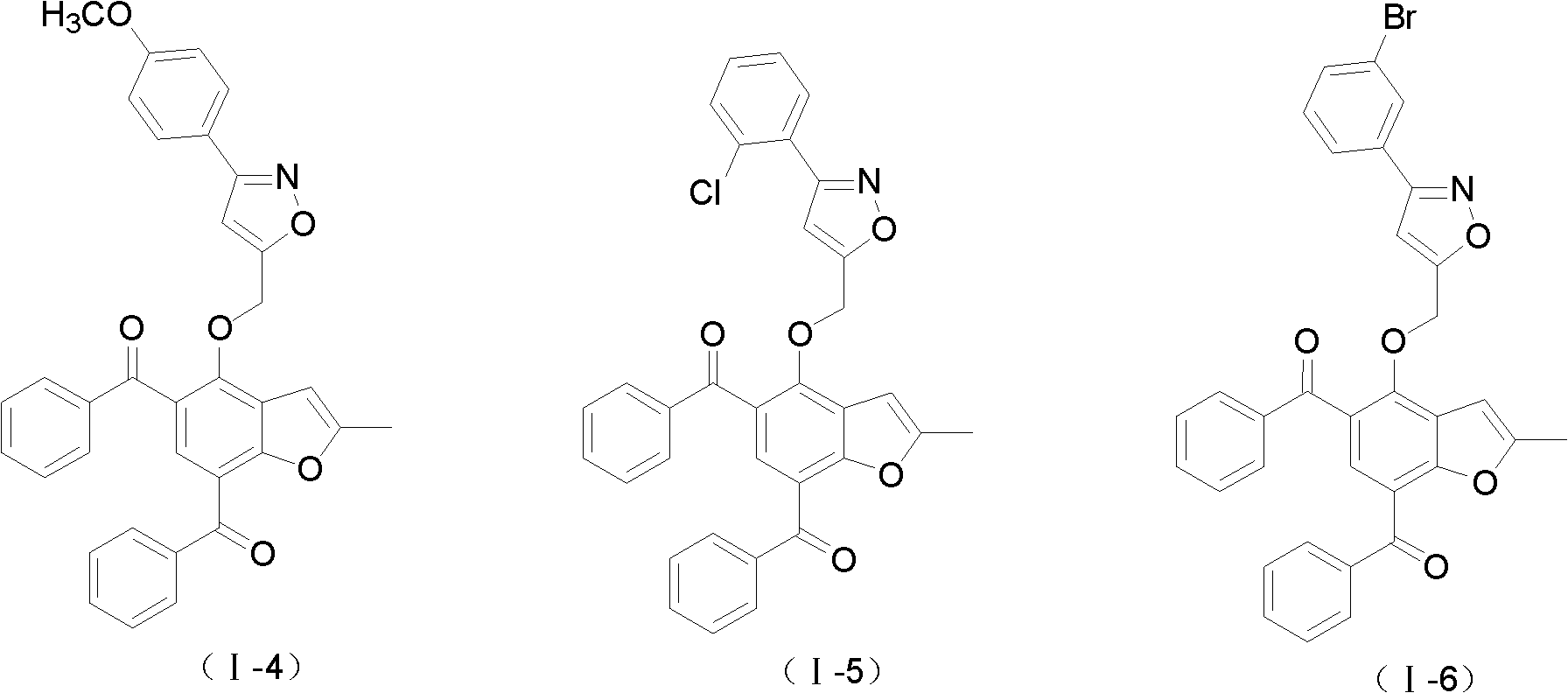
2-methylbenzofuran derivative containing isoxazole heterocycle, and preparation method and application thereof
A technology of isoxazole heterocycle and methylbenzene, which is applied in the field of 2-methylbenzofuran derivatives, can solve the problems of impossibility and time-consuming, improve efficiency, increase reaction yield, and simplify post-reaction treatment The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of selenium resin load (IV-1)
[0045] The reaction formula is as follows:
[0046]
[0047] Under a nitrogen atmosphere, 0.8 grams of 1.25mmol / g (1.00mmol) polystyrene-loaded selenium bromide resin (II) in 30mL CHCl 3 Soak in the middle for 12 hours, then add 1.79g (5.0mmol) compound shown in formula (III-1), 0.580g (5.0mmol) tetramethylethylenediamine, stir and react at 60°C for 4.0 hours, filter the reaction solution, filter The cake was successively filled with 20mL of water, 20mL of tetrahydrofuran, 20mL of tetrahydrofuran and water mixture (volume ratio of 1:1), 20mL of tetrahydrofuran, 20mL of tetrahydrofuran and water mixture (volume ratio of 1:1), 20mL of tetrahydrofuran, 20mL of chloroform, 20mL Washed with dichloromethane, dried under vacuum at 45° C. for 6 hours to obtain 1.076 g of the selenium resin supported substance represented by formula (IV-1).
Embodiment 2
[0048] Embodiment 2: Preparation of selenium resin load (V-1)
[0049] The reaction formula is as follows:
[0050]
[0051] Under a nitrogen atmosphere, 1.076 g of the selenium resin load (IV-1) obtained in Example 1 (the amount of the material loaded with selenium is 1.0 mmol) was soaked in 30 mL of chloroform for 5 hours, and 0.75 mL (8.0 mmol) of alkyne was added thereto. Propyl bromide, 1.12g (8.0mmol) potassium carbonate, reacted at 60°C for 8.0 hours, filtered the reaction solution, and washed the filter cake with 30mL of water, 30mL of tetrahydrofuran, 30mL of tetrahydrofuran and water mixture (volume ratio: 1:1), Wash with 30mL tetrahydrofuran, 30mL tetrahydrofuran and water mixture (1:1 volume ratio), 30mL tetrahydrofuran, 30mL chloroform, and 30mL dichloromethane, and vacuum-dry at 40°C for 6 hours to obtain the selenium resin-loaded solution shown in formula (V-1). Material 1.110g.
Embodiment 3
[0052] Embodiment 3: The preparation reaction formula of 2-methylbenzofuran derivative (I-1) containing isoxazole heterocycle is as follows:
[0053]
[0054]
[0055] (1) Under a nitrogen atmosphere, 0.54g (4.0mmol) of 4-methylbenzaldehyde oxime (VI-1) was reacted in methylene chloride at 30°C for 4 hours, and then soaked in methylene chloride for 5 The selenium resin loading (V-1) 1.110g (the amount of the material of loading selenium is 1.0mmol) prepared by the embodiment 2 method of 1 hour mixes, stirs, then in the mixture, dropwise add 0.70mL (5.0mmol) triethylamine , reacted for 18 hours at 25°C, ended the reaction, filtered the reaction solution, and the filter cake was successively washed with 40mL of water, 40mL tetrahydrofuran, 40mL tetrahydrofuran and water mixed solution (volume ratio is 1: 1), 40mL tetrahydrofuran, 40mL tetrahydrofuran and water mixed solution ( The volume ratio is 1:1), 40mL tetrahydrofuran, 40mL chloroform, 40mL dichloromethane washing, 40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com