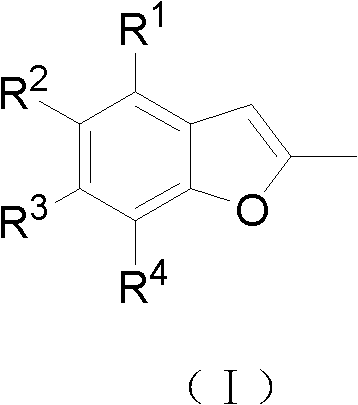
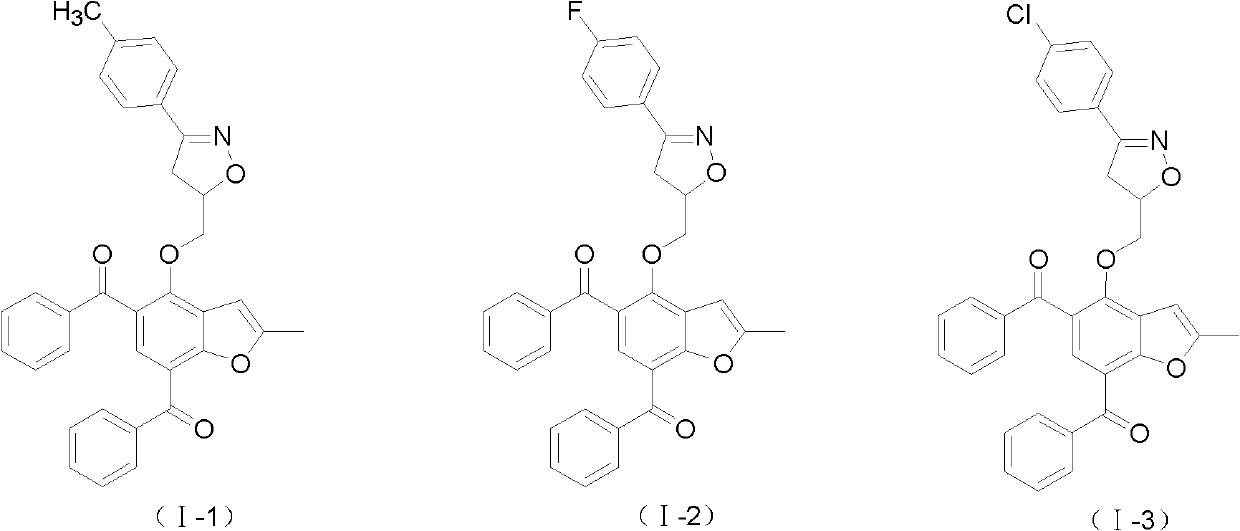
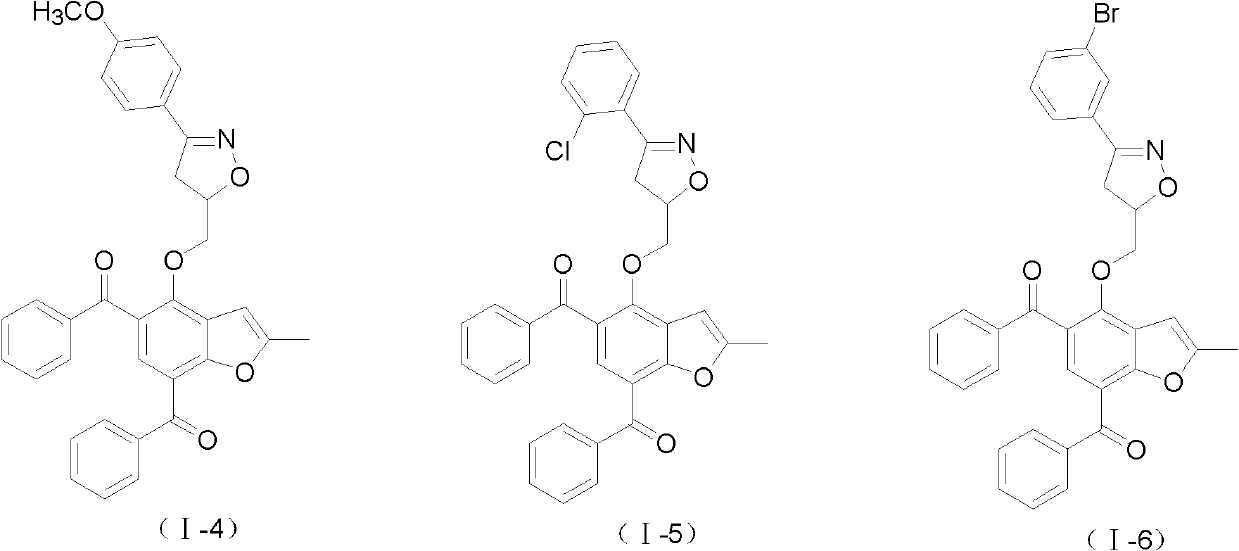
2-methyl-benzofuran compounds and preparation and application thereof
A compound and methylbenzene technology, applied to 2-methylbenzofuran compounds and their preparation and application fields, can solve problems such as impossibility, time-consuming, etc., and achieve the advantages of improving efficiency, improving reaction yield, and realizing automatic operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the preparation of selenium resin load (IV-1)
[0048] The reaction formula is as follows:
[0049]
[0050] Under a nitrogen atmosphere, 0.8 grams of 1.25mmol / g (selenium loading 1.00mmol) of polystyrene-loaded selenium bromide resin (II) in 30mLCHCl 3 Soak in the middle for 12 hours, then add 1.79g (5.0mmol) compound shown in formula (III-1), 0.580g (5.0mmol) tetramethylethylenediamine, stir and react at 60°C for 4.0 hours, filter the reaction solution, filter The cake was successively filled with 20mL of water, 20mL of tetrahydrofuran, 20mL of tetrahydrofuran and water mixture (volume ratio of 1:1), 20mL of tetrahydrofuran, 20mL of tetrahydrofuran and water mixture (volume ratio of 1:1), 20mL of tetrahydrofuran, 20mL of chloroform, 20mL Washed with dichloromethane, dried under vacuum at 45° C. for 6 hours to obtain 1.076 g of the selenium resin supported substance represented by formula (IV-1).
Embodiment 2
[0051] Embodiment 2: Preparation of selenium resin load (XII-1)
[0052] Under a nitrogen atmosphere, 1.076 g of the selenium resin load (IV) prepared by the method in Example 1 (the amount of the material loaded with selenium is 1.0 mmol) was soaked in 30 mL of chloroform for 5 hours, and 1.21 g (10.0 mmol) of alkene was added thereto. Propyl bromide, 1.12g (8.0mmol) potassium carbonate, reacted at 60°C for 8.0 hours, filtered the reaction solution, and washed the filter cake with 40mL of water, 40mL of tetrahydrofuran, 40mL of tetrahydrofuran and water mixture (volume ratio: 1:1), 40mL tetrahydrofuran, 40mL tetrahydrofuran and water mixed solution (volume ratio is 1:1), 40mL tetrahydrofuran, 40mL chloroform, 40mL dichloromethane wash, 40 ℃ of vacuum drying, obtain the selenium resin load 1.112 shown in formula (XII-1) g.
[0053] The reaction formula is as follows:
[0054]
Embodiment 3
[0055] Embodiment 3: Preparation of 2-methylbenzofuran compound (I-1)
[0056] The reaction formula is as follows:
[0057]
[0058] (1) Under a nitrogen atmosphere, 0.54g (4.0mmol) of 4-methylbenzaldehyde oxime (XIII-1) was reacted in 15mL of dichloromethane at 30°C for 4 hours, and soaked in 20mL of dichloromethane beforehand. After 5 hours, the selenium resin load (XII-1) 1.112g (the amount of the material loaded with selenium is 1.0mmol) prepared by the method of Example 2 was mixed, stirred, and then 0.70mL (5.0mmol) three Ethylamine was reacted at 25°C for 18 hours, the reaction was terminated, the reaction solution was filtered, and the filter cake was mixed with 40mL of water, 40mL of tetrahydrofuran, 40mL of tetrahydrofuran and water mixture (volume ratio of 1:1), 40mL of tetrahydrofuran, 40mL of tetrahydrofuran and water. solution (volume ratio 1:1), 40 mL tetrahydrofuran, 40 mL chloroform, 40 mL dichloromethane, and vacuum-dried at 40° C. for 6 hours to obtain 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com