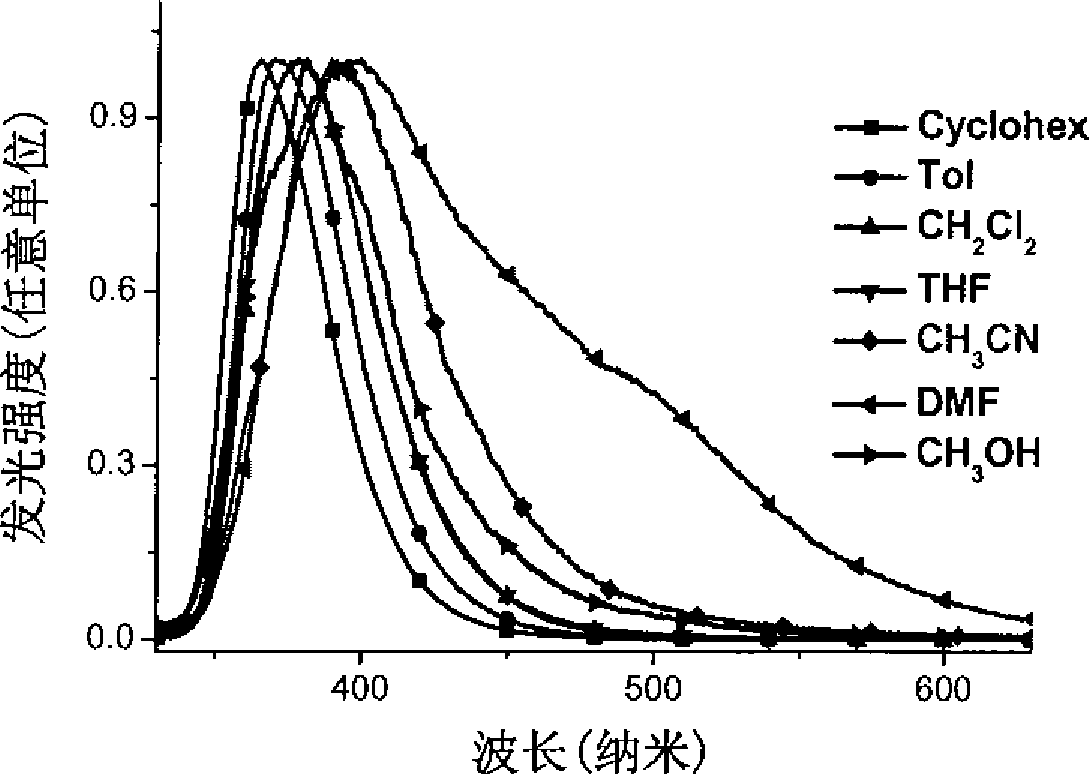
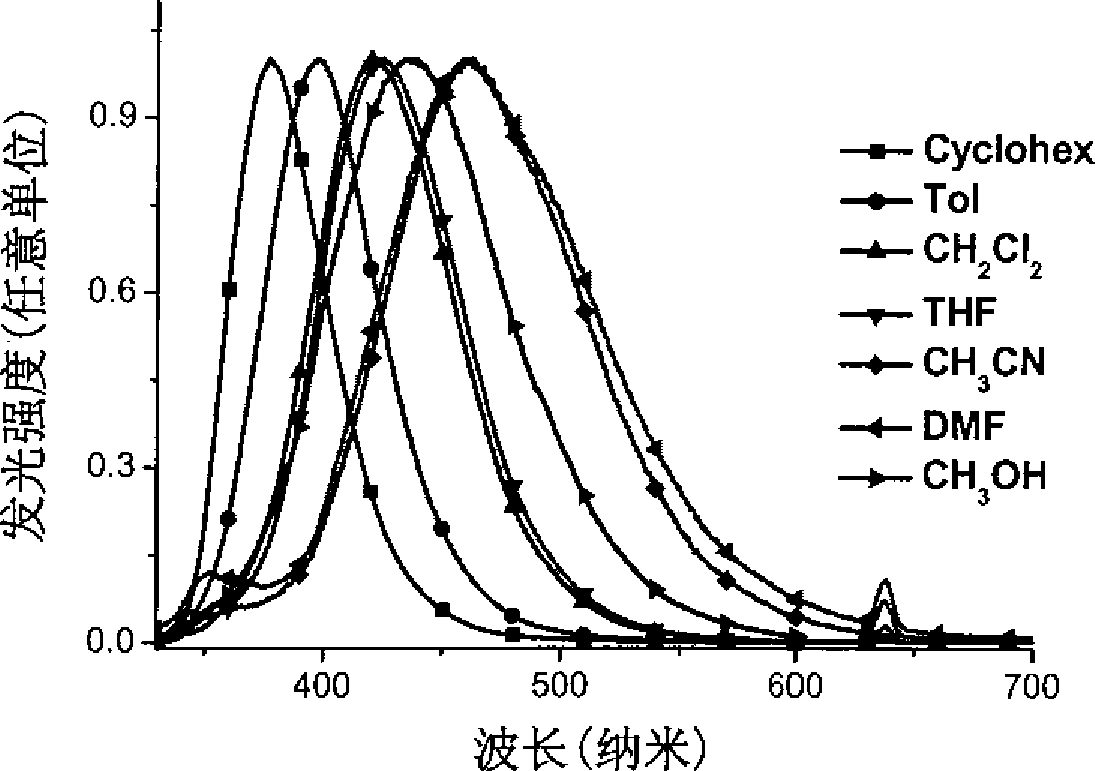
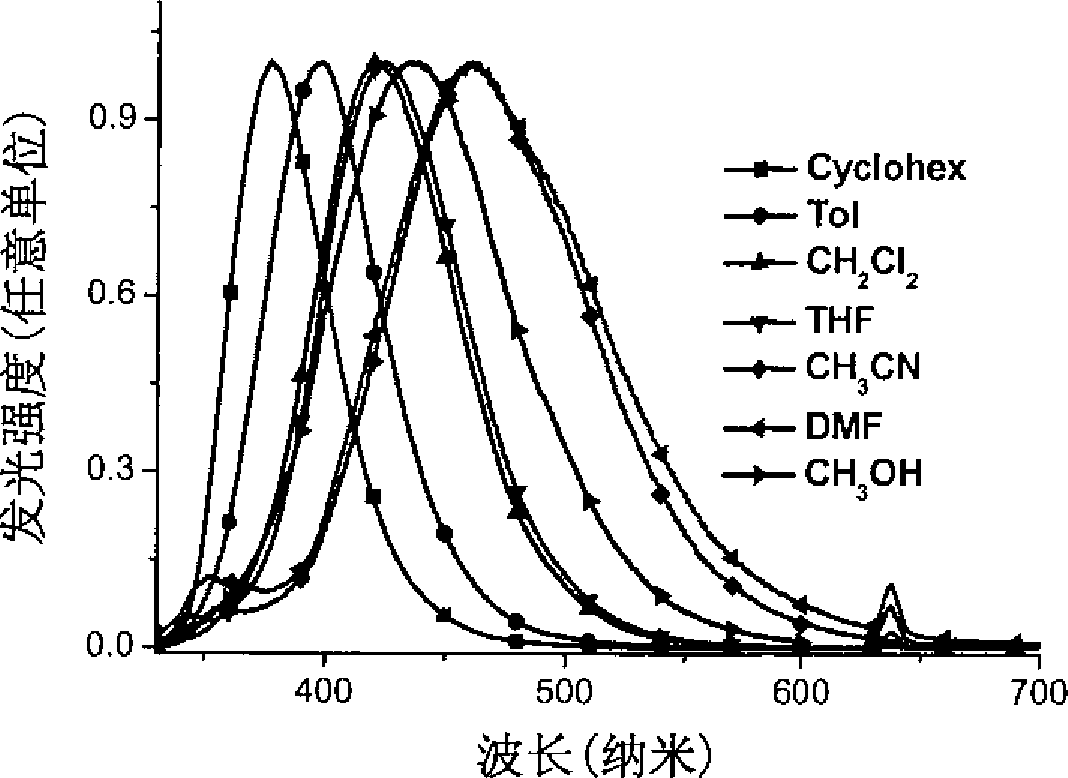
Star-shaped triaryl borane compounds as well as preparation method and application thereof
A technology of star-shaped triarylborane and compound, applied in the field of fluorescent probes, can solve the problems of low reaction sensitivity, poor selectivity, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of 1,2-bis(4-(bis(2,4,6-trimethylphenyl)boryl)phenyl)acetylene (B-B)
[0067]
[0068] Add 500mg (1.487mmol) of bis(4-bromophenyl)acetylene and 10mL of freshly distilled tetrahydrofuran into a 25mL Schlenk bottle. After removing water and oxygen, place the system in a low-temperature bath at -78°C, and add 1.8mL of 2. 2M (3.96mmol) n-BuLi solution. Keep at -78°C, stir the reaction for 2 hours, and add 4 mL of 808 mg (3.01 mmol) bis(2,4,6-trimethylphenyl) boron fluoride-tetrahydrofuran (THF) solution dropwise. After the dropwise addition, the reaction system naturally rose to room temperature and reacted at room temperature for 24 hours. The reaction was quenched by adding water, THF was removed by rotary evaporation, and the aqueous phase was extracted three times with dichloromethane, and the organic phases were combined and washed three times with saturated brine and water respectively. The organic phase was dried with anhydrous sodium sulfate, spin-d...
Embodiment 2
[0070] Preparation of bis(4-(9-carbazole)phenyl)acetylene and 1-(4-bromophenyl)-2-(4-(9-carbazole)phenyl)acetylene
[0071]
[0072] Add 102.6mg (0.305mmol) bis(4-bromophenyl) acetylene, 130.3mg (0.799mmol) carbazole, 170mg (1.232mmol) anhydrous potassium carbonate, 38mg (0.594mmol) activated copper powder in a 50mL two-necked flask , 52mg (0.197mmol) of 18-crown-6 and 8mL of o-dichlorobenzene were passed through nitrogen for 15min, heated to 180°C under nitrogen atmosphere, and refluxed for 24h. The reaction system was cooled to room temperature, filtered under reduced pressure, and the filtrate was spin-dried, and separated by using petroleum ether / dichloromethane as an eluent chromatographic column (200-300 mesh silica gel) to obtain a white solid, bis(4-(9- Carbazole)phenyl)acetylene 45mg, yield 30%; 1-(4-bromophenyl)-2-(4-(9-carbazole)phenyl)acetylene 60mg, yield 46.6%. Increase the proportion of carbazole (>6eq), mainly to obtain bis(4-(9-carbazole)phenyl)acetylene; ...
Embodiment 3
[0076] Preparation of 1-(4-(bis(2,4,6-trimethylphenyl)boryl)phenyl)-2-(4-(9-carbazole)phenyl)acetylene (B-C)
[0077]
[0078] Add 155 mg (0.368 mmol) 1-(4-bromophenyl)-2-(4-(9-carbazole) phenyl) acetylene and 30 mL freshly distilled tetrahydrofuran into a 50 mL schlenk bottle, repeat vacuum pumping-nitrogen three times, and In a low-temperature bath at -78°C, 0.3mL of 2.2M (0.7mmol) n-butyllithium solution was added dropwise under a nitrogen atmosphere. Keep the low-temperature bath at -78°C, stir the reaction for 2 hours, and add 2 mL of 148 mg (0.558 mmol) bis(2,4,6-trimethylphenyl) boron fluoride-tetrahydrofuran solution dropwise. After the dropwise addition, the reaction system was allowed to rise to room temperature naturally, and reacted at room temperature for 24 h. The reaction was quenched by adding water, THF was removed by rotary evaporation, the aqueous phase was extracted three times with dichloromethane, the organic phases were combined, and washed three tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com