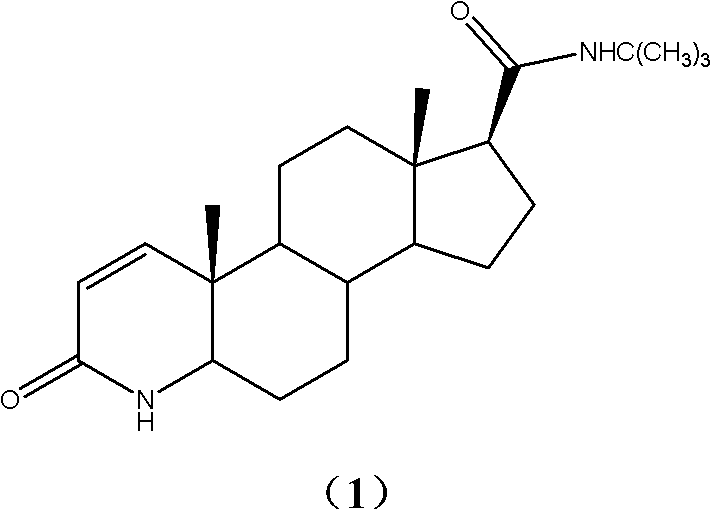
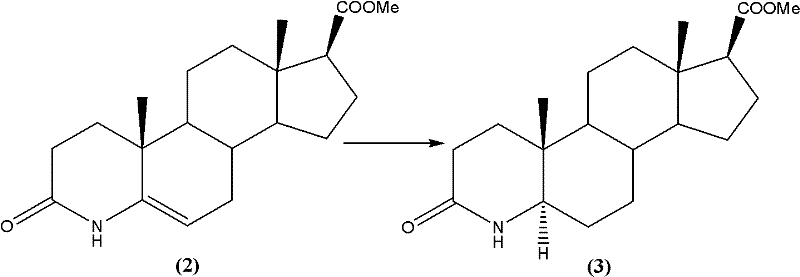
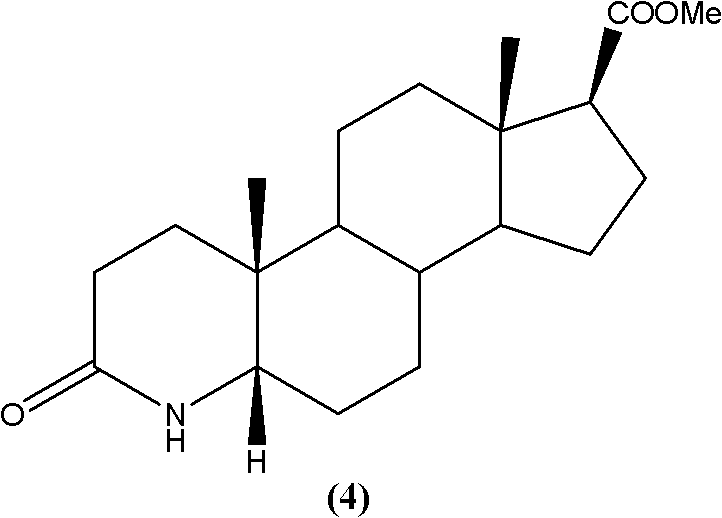
Synthetic method of 3-carbonyl-4-aza-5 alpha-androstane-17 beta-carboxylic acid methyl ester
A technology of methyl carboxylate and synthesis method, which is applied in the field of synthesis of 3-carbonyl-4-aza-5α-androst-17β-methyl carboxylate, which can solve the problem that isomers cannot be recycled and applied mechanically, which limits industrial production , Difficult purification and separation, etc., to achieve the effect of facilitating industrial implementation, avoiding reaction condition problems, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of 3-carbonyl-4-aza-5α-androst-17β-carboxylate methyl ester:
[0023] Add 3-carbonyl-4-aza-5-androstene-17β-carboxylate methyl ester (33.14g; Fw: 331.45; 100 mmol), followed by the addition of 200 mL of ethanol and 3.31 g of Raney-Ni. Then 60 g of triethylamine formate were added. The system continued to stir for 2h under reflux. After the reaction was completed, after the system was cooled to room temperature, the solid was removed by filtration, and the filtrate was evaporated to dryness. After adding ice water, it was extracted with dichloromethane. The extract was washed and dried, and evaporated to dryness to obtain a crude product. The α-isomer in the crude product was analyzed by HPLC. : β isomer>99:1, the product was subsequently recrystallized from a solvent to obtain 31.67 g of the target product, yield: 95%, HPLC content greater than 99%.
Embodiment 2
[0025] Preparation of 3-carbonyl-4-aza-5α-androst-17β-carboxylate methyl ester
[0026] Add 3-carbonyl-4-aza-5-androstene-17β-carboxylate methyl ester (33.14g; Fw: 331.45; 100 mmol), followed by the addition of 200 mL of methanol and 3.31 g of Raney-Ni. Then 60 g of triethylamine formate were added. The system continued to stir for 5h under reflux. After the reaction was completed, after the system was cooled to room temperature, the solid was removed by filtration, and the filtrate was evaporated to dryness. After adding ice water, it was extracted with dichloromethane. The extract was washed and dried, and evaporated to dryness to obtain a crude product. The α-isomer in the crude product was analyzed by HPLC. : β isomer>98:2, in the product, the product was then recrystallized from a solvent to obtain 31.67 g of the target product, yield: 95%, and the HPLC content was greater than 99%.
Embodiment 3
[0028] Preparation of 3-carbonyl-4-aza-5α-androst-17β-carboxylate methyl ester
[0029] Add 3-carbonyl-4-aza-5-androstene-17β-carboxylate methyl ester (33.14g; Fw: 331.45; 100 mmol), followed by the addition of 200 mL of methanol and 0.33 g of Raney-Ni. Then 35 g of triethylamine formate were added. The system continued to stir for 24h under reflux. After the reaction was completed, after the system was cooled to room temperature, the solid was removed by filtration, and the filtrate was evaporated to dryness. After adding ice water, it was extracted with dichloromethane. The extract was washed and dried, and evaporated to dryness to obtain a crude product. The α-isomer in the crude product was analyzed by HPLC. : β isomer>97:3, in the product, the product was then recrystallized from a solvent to obtain 31.67 g of the target product, yield: 95%, and the HPLC content was greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com