Coal gas high-temperature methanation catalyst and preparation method thereof
A methanation catalyst, coal-to-natural gas technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem that the catalyst does not have high temperature resistance and pressure resistance. and other problems, to achieve the effect of improving high temperature hydrothermal stability, long life, high activity, and reducing the amount of Ni
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
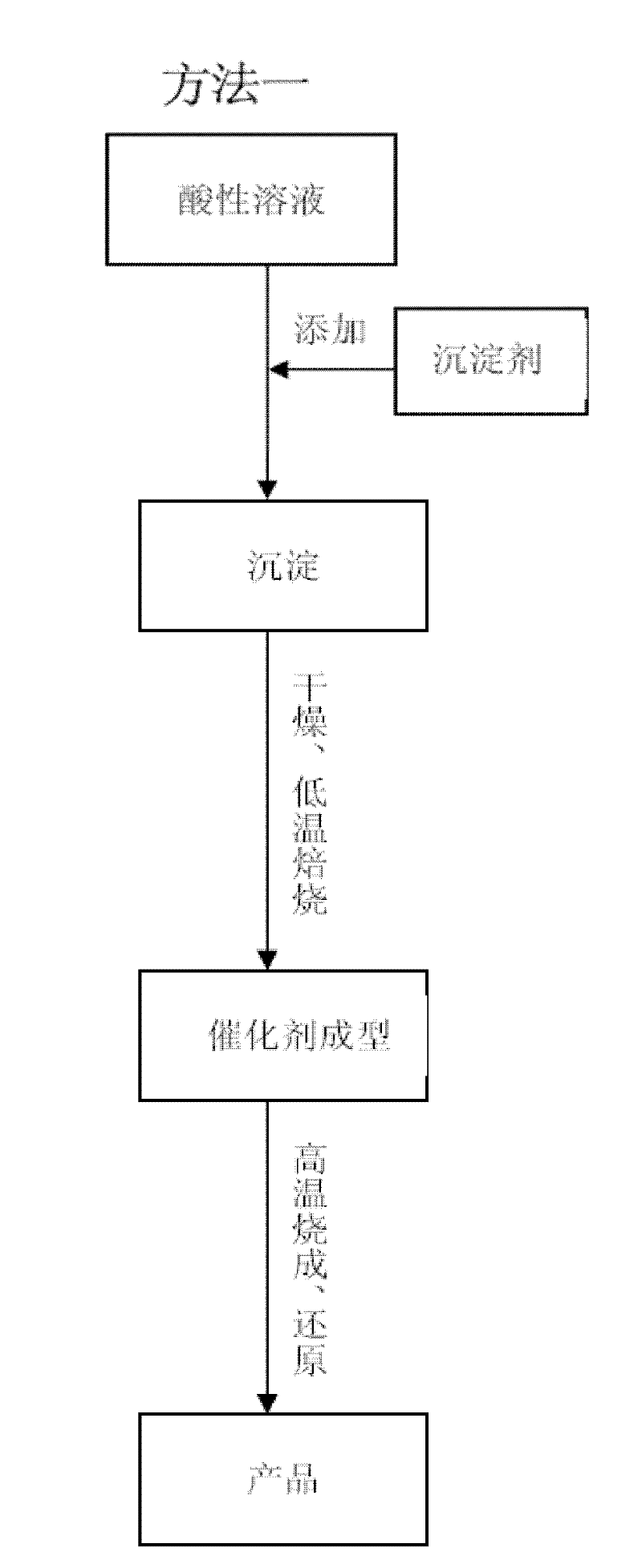
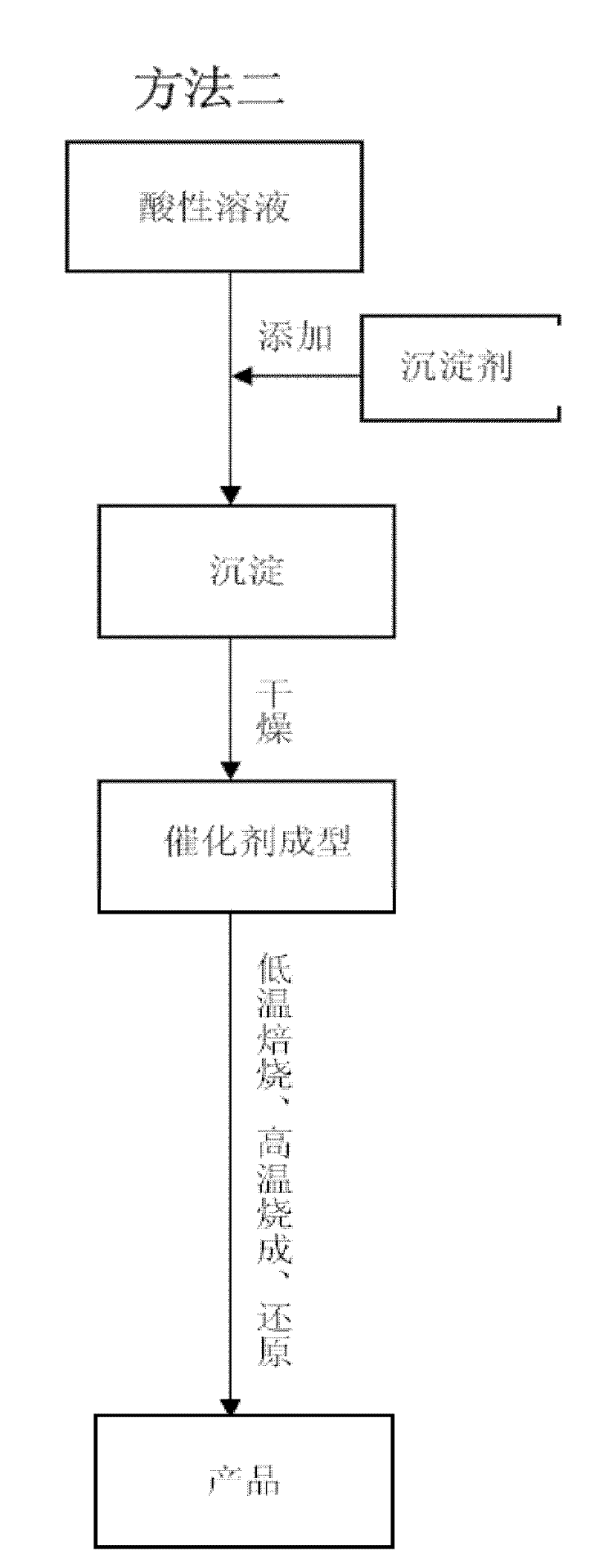
[0033] In the preparation method of the present invention, the active component that comprises catalyst, the various metal elements of support, as the soluble compound of Ni, Fe, Al, Zr etc. is nitrate, sulfate, hydrochloride, these strong acid salts The aqueous solution itself is acidic and can provide free metal ions; some soluble or insoluble compounds are carbonates, oxides, hydroxides, and hydrates of oxides and their minerals, such as monohydrate alumina, trihydrate oxide Aluminum, basic zirconium carbonate, etc., can be acidified by aqueous solutions such as nitric acid, sulfuric acid, hydrochloric acid, or the aqueous solution of the above-mentioned acidic soluble compounds, so that all or part of the metal elements contained in them can be dissolved in the form of metal ions. in the water. In order to avoid residues of heteroatoms such as sulfur and chlorine, nitrates are preferred for soluble salts, nitric acid is preferred for acidification treatment, and carbonates...
Embodiment 1
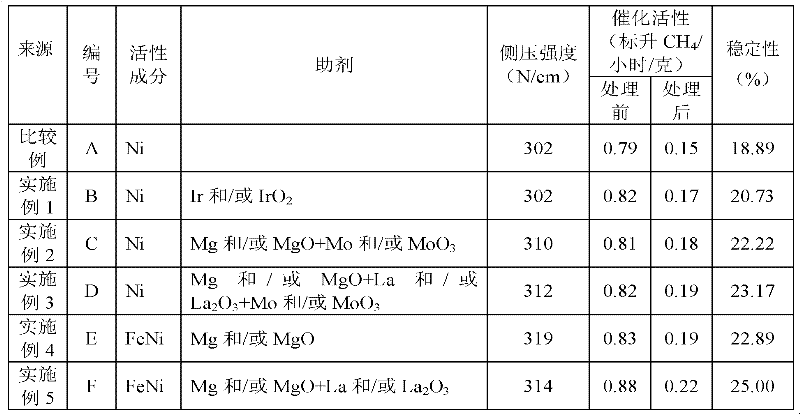
[0044] Take 37.7 grams of Ni(NO 3 ) 2 ·6H 2 O, 112.5 g Al(NO 3 )3 9H 2 O, 26.8 grams of Zr(NO 3 ) 4 ·5H 2 O, 0.3 g Ir(NO 3 ) 4 , dissolved in 1000ml deionized water. Over 20 minutes, 103.2 g of ammonium bicarbonate were added slowly, followed by stirring for a further 2 hours. The suspension was filtered and washed with 2000 ml of deionized water to obtain a light green precipitate. The filter cake was dried with hot air at 120° C. for 20 hours, and roasted at 400° C. for 16 hours to obtain 32.6 grams of a roasted sample. Crushed and passed through a 100-mesh sample sieve, mixed with graphite and cellulose and pressed to obtain cylindrical (Φ3) catalyst particles. After firing at 1100°C for 3 hours, the catalyst B in the oxidation state was obtained, in which, IrO 2 The content is 0.5%.
Embodiment 2
[0046] Take 37.7 grams of Ni(NO 3 ) 2 ·6H 2 O, 112.5 g Al(NO 3 ) 3 9H 2 O and 26.8 g of Zr(NO 3 ) 4 ·5H 2 O, 20.8 g Mg(NO 3 ) 2 ·6H 2 O, dissolved in 1000ml of water to obtain a green clear solution. Take MoO 3 Content is 1.0 grams of ammonium molybdate dissolved in 250 ml of aqueous solution prepared by 103.2 grams of ammonium bicarbonate, and cocurrent precipitation with the aforementioned nitrate solution as an acidic solution. After aging for 3 hours, the precipitate was filtered and washed with 2000 ml of water. After the filter cake was dried with hot air at 120°C for 20 hours, it was crushed and passed through a 100-mesh sample sieve. After adding scallop powder, oxalic acid and water, it was extruded to obtain strip-shaped (Φ3) catalyst particles. Calcined at 400°C for 16 hours and at 1100°C for 3 hours to obtain catalyst C in oxidation state, in which MgO and MoO 3 The contents were 8.8% and 2.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com