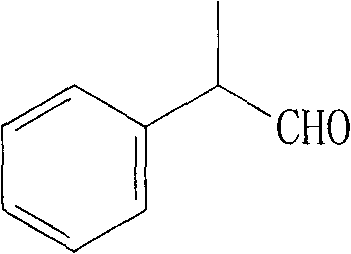
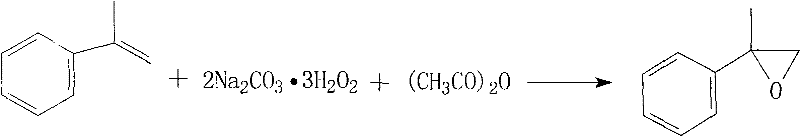Method for producing hydratropic aldehyde
A production method, the technology of solanum aldehyde, applied in the production field of solanum aldehyde, can solve the problems of unfavorable industrial production, instability of peracetic acid, harsh reaction conditions, etc., and achieve the effect of low cost, few reaction steps and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 2L three-necked flask equipped with a thermometer, stirring, dropping funnel and condensation column, add 800g of toluene, 180g of α-methylstyrene, and 650g of sodium percarbonate, stir vigorously, raise the temperature to 55°C, and slowly drop in acetic anhydride 300g, continue the reflux reaction after the dropwise addition, follow the reaction by chromatography, until the complete conversion of α-methylstyrene, the reaction time is about 25 to 30 hours, filter off the solid inorganic matter, wash to remove the residual peroxide, and recover the solvent Rectifying to obtain 2-phenylpropylene oxide with a content of ≥98%, and the yield is 90-95%.
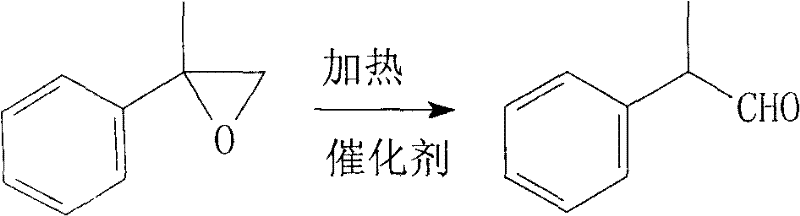
[0023] In a 500ml three-neck flask equipped with a thermometer, stirring, dropping funnel, and condensation column, add 200g of toluene, stir and raise the temperature to 130°C, and slowly add 120g of the above-prepared 2-phenylpropylene oxide dropwise. Continue the reflux reaction, follow the reaction by chromatography...
Embodiment 2
[0025] In a 2L three-neck flask equipped with a thermometer, stirring, dropping funnel, and condensation column, add 1000 g of dichloromethane, 140 g of α-methylstyrene, 600 g of sodium percarbonate, and 8 g of triethylbenzyl ammonium chloride. Stir, raise the temperature to 45°C, slowly add 200g of acetic anhydride dropwise, continue the reflux reaction after the dropwise addition, follow the reaction by chromatography, until the complete conversion of α-methylstyrene, the reaction time is about 18 to 22 hours, filter off the solid inorganic matter, Washing with water to remove the residual peroxide, recovering the solvent and rectifying to obtain 2-phenylpropylene oxide with a content of ≥98% and a yield of 85-90%.
[0026] In a 500ml three-neck flask equipped with a thermometer, stirring, dropping funnel and condensation column, add 200g of benzene and 2g of zinc chloride, stir and raise the temperature to 60°C, and slowly drop in 140g of the above-prepared 2-phenylpropylene...
Embodiment 3
[0028] In a 500ml three-neck flask equipped with a thermometer, stirring, dropping funnel, and condensation column, add 200g of benzene, 40g of α-methylstyrene, 150g of sodium percarbonate, and 6g of polyethylene glycol 600, stir vigorously, and heat up to 42°C , slowly drop 60g of acetic anhydride, continue to reflux reaction after the dropwise addition, follow the reaction by chromatography, until the complete conversion of α-methylstyrene, the reaction time is about 15 to 20 hours, filter off the solid inorganic matter, wash with water to remove the residual excess Oxides, recovering the solvent and rectifying to obtain 2-phenylpropylene oxide with a content of ≥98%, and the yield is 90-95%.
[0029] In a 500ml three-neck flask equipped with a thermometer, stirring, dropping funnel and condensation column, add 200g of benzene, 2g of zinc bromide, stir and raise the temperature to 80°C, and slowly drop in 140g of the above-prepared 2-phenylpropylene oxide , continue the refl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com