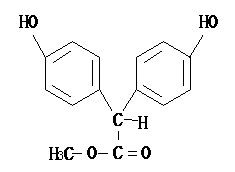
2-(4-hydroxy benzene)-methyl acetate and its preparation method
A methyl acetate, hydroxybenzene technology, applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds and other directions, can solve the problems of broad antibacterial spectrum and narrow, weak antibacterial ability, harmful to health, etc. Bacteriostatic effect, no pollutant discharge, mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, the preparation method of 2-(4-hydroxybenzene)-methyl acetate comprises the following steps:
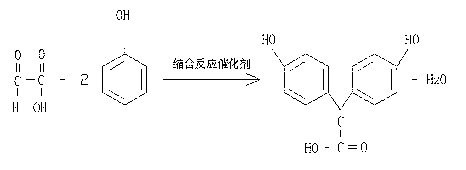
[0029] Condensation reaction: Add 122.2g of condensation reaction catalyst concentrated sulfuric acid and 136.3g of deionized water into a 5000ml four-necked reaction flask, add 470g of phenol under normal temperature and pressure conditions, there is a small amount of insoluble matter, start stirring, and dropwise add 180.8g of glyoxylic acid , the color of the reaction solution gradually deepens. After the addition is completed, the reaction solution is light red. The reaction is carried out at a temperature of 25-30°C for 18-20 hours. After TLC detection and tracking, the spots of the raw materials disappear and reach the end point, that is, the reaction is complete. The chemical reaction formula is as follows:
[0030] ;
[0031] Neutralization and extraction: After the condensation reaction is completed, add 800g of cold water and 280g of sodium bicarb...
Embodiment 2
[0040] Embodiment 2, the preparation method of 2-(4-hydroxybenzene)-methyl acetate comprises the following steps:
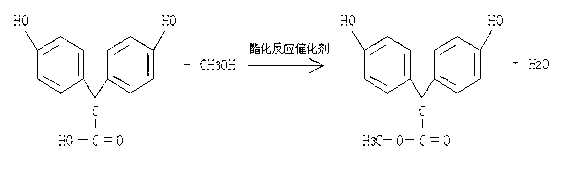
[0041] Condensation reaction: Add 164.5g of condensation reaction catalyst concentrated sulfuric acid and 155.1g of deionized water into a 5000ml four-necked reaction flask, add 470g of phenol under normal temperature and pressure conditions, there is a small amount of insoluble matter, start stirring, and dropwise add 151.6g of glyoxylic acid , the color of the reaction solution gradually deepens. After the addition is completed, the reaction solution is light red. The reaction is carried out at a temperature of 35-40°C for 18-20 hours. TLC detection and tracking, the spots of the raw materials disappear and reach the end point, that is, the reaction is complete. The reaction formula is as follows:
[0042] ;
[0043] Neutralization and extraction: After the condensation reaction is completed, at normal pressure and at a temperature of 25±5°C, adjust the pH to...
Embodiment 3
[0052] Embodiment 3, the preparation method of 2-(4-hydroxybenzene)-methyl acetate comprises the following steps:
[0053] Condensation reaction: Add 141g of condensation reaction catalyst concentrated sulfuric acid and 145.7g of deionized water into a 5000ml four-necked reaction flask, add 470g of phenol under normal temperature and pressure conditions, there is a small amount of insoluble matter, start stirring, and add 167.9g of glyoxylic acid dropwise, The color of the reaction solution gradually deepens. After the addition is completed, the reaction solution is light red. The reaction is carried out at a temperature of 30-35°C for 20 hours. After TLC detection and tracking, the spots of the raw materials disappear and reach the end point, that is, the reaction is complete. The reaction formula is as follows:
[0054] ;
[0055] Neutralization and extraction: After the condensation reaction is over, add 900g of cold water and 300g of sodium bicarbonate to the system at n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com