Lipid-water amphiphilic benzylidene cyclopentanone dye and preparation method and application in photodynamic therapy thereof
A technology of groups and methyl groups is applied in the field of preparation of lipid-water amphiphilic benzylidene cyclopentanone dyes, which can solve the problems of slow metabolism in vivo, low killing efficiency, unclear effective components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] (i) Add 8 grams (0.2 mol) of NaOH and 40 milliliters of water into a 250 milliliter three-neck flask, stir to make it dissolve evenly. 14.42 g (0.12 mol) of dipolyethylene glycol monomethyl ether was added into 30 ml of tetrahydrofuran (THF) to dissolve evenly, and then added to the above-mentioned three-necked flask and mixed evenly with NaOH solution. Mix 22.88 grams (0.12 mol) of p-toluenesulfonyl chloride and 40 milliliters of THF evenly, and then slowly drop them into the above-mentioned three-necked flask. During the dropping process, keep the temperature of the reaction solution not exceeding 10°C. After the dropwise addition, continue to stir and react for 4 hours. Stop responding. The reaction solution was extracted three times with diethyl ether, the extract was washed with water until it was neutral, then dried by adding anhydrous sodium sulfate, filtered and rotary evaporated to remove diethyl ether to obtain 30.2 g (yield: 92%) of the corresponding p-toluen...
Embodiment 2
[0101] (i) Add 8 grams (0.2 mol) of NaOH and 80 milliliters of water into a 250 milliliter three-neck flask, stir to make it dissolve evenly. 19.7 g (0.12 mol) of tripolyethylene glycol monomethyl ether was added into 50 ml of THF to dissolve evenly, and then added to the above-mentioned three-necked flask and mixed evenly with NaOH solution. Mix 22.88 grams (0.12 mol) of p-toluenesulfonyl chloride and 40 milliliters of THF evenly, and then slowly drop them into the above-mentioned three-necked flask. During the dropping process, keep the temperature of the reaction solution not exceeding 10°C. After the dropwise addition, continue to stir and react for 6 hours. Stop responding. The reaction solution was extracted three times with diethyl ether, the diethyl ether extract was washed with water until it was neutral, and then dried by adding anhydrous magnesium sulfate. The diethyl ether was removed by filtration and rotary evaporation to obtain 34.7 g (yield: 91%) of the corresp...
Embodiment 3
[0110] (i) With reference to the operation of (v) in Example 1, use p-toluenesulfonate Al and 4-(N-methyl-N-(2-hydroxyl-ethyl)amino)benzene with a molar weight of 1:1 formaldehyde reaction to prepare the corresponding benzaldehyde derivative E3 with a PEG group, the yield is 75%, 1 HNMR (400MHz CDCl 3 ): δ(ppm) 3.07(s, 3H), 3.34(s, 3H), 3.51~3.65(m, 12H), 6.71(d, J=9Hz, 2H), 7.69(d, J=9Hz, 2H) , 9.70 (s, 1H).
[0111]
[0112] (ii) With reference to the operation of (vi) in Example 1, the target dye H3 was prepared by reacting E3 with a molar mass of 2:1 with cyclopentanone, with a yield of 72%, 1 HNMR (400MHz CDCl 3 ): δ (ppm) 3.05 (s, 6H) 3.08 (s, 4H) 3.37 (s, 6H) 3.51 ~ 3.67 (m, 24H) 6.73 (d, J = 8.6Hz, 4H) 7.51 (d, J = 8.6 Hz, 6H). HR-MS (ESI): m / z Calcd for C 35 h 51 N 2 o 7 [M+H] + 611.36908; found 611.36926.
[0113]
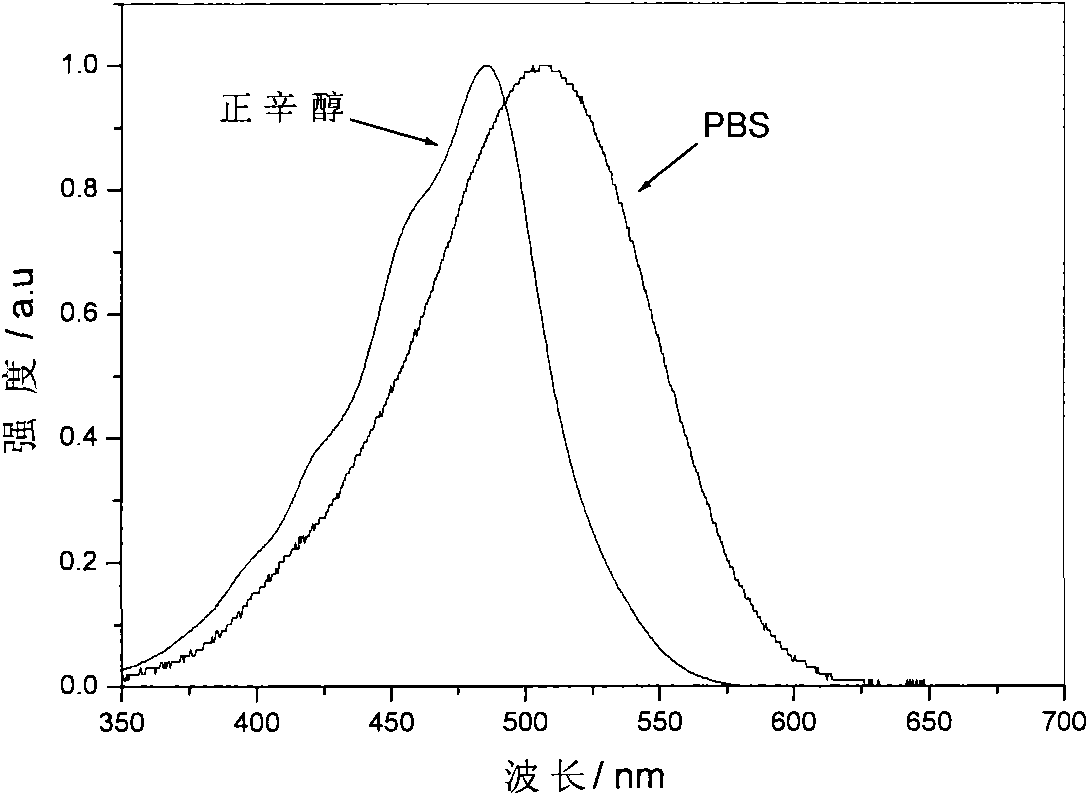
[0114] (iii) Use PBS (pH=7.4) buffer solution to detect the solubility of the target dye H3 in the water system, and its maximum solubil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com