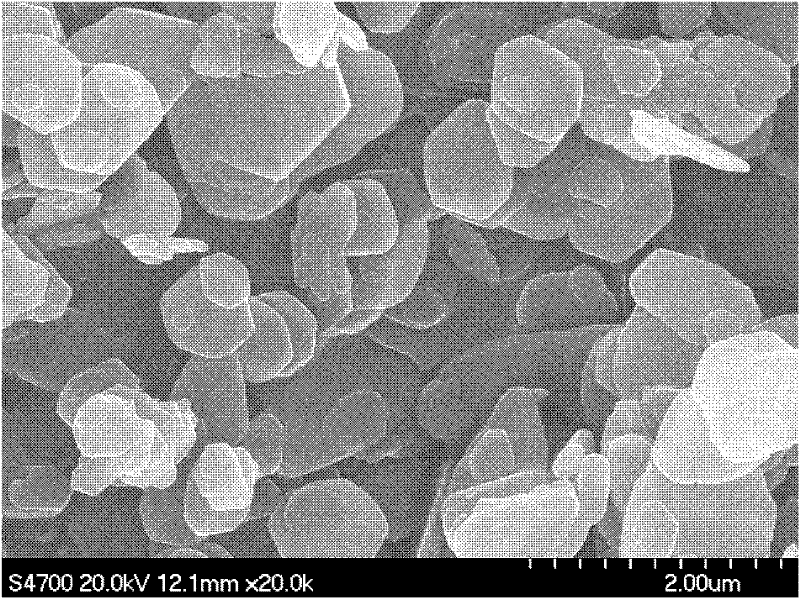
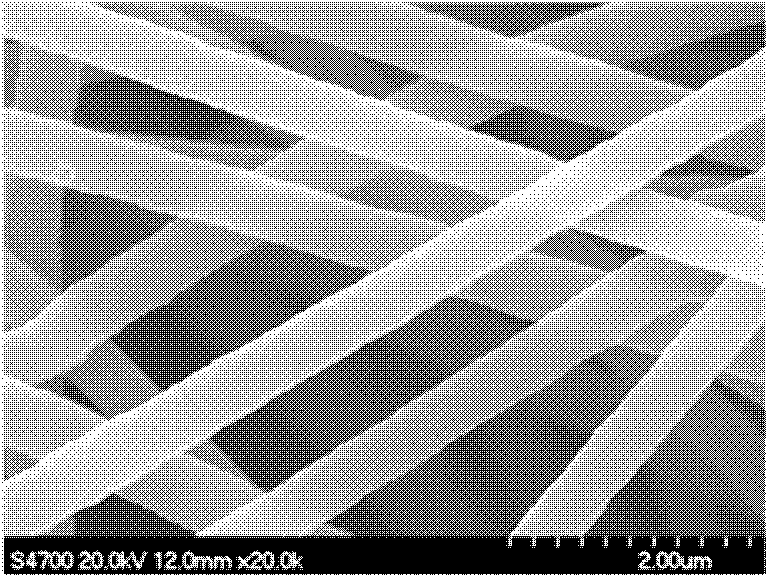
Method for preparing magnesium hydroxide utilizing light-burned dolomite powder
A light-burned dolomite powder and magnesium hydroxide technology, which is applied in the direction of magnesium hydroxide, can solve the problems of difficult control of the ratio of strong alkali and uneven structure of magnesium hydroxide, and achieve easy control of reaction conditions, environmental protection, and high purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) Pour 60g of light-burned dolomite powder into 1 liter of water at 70°C and digest it for 40 minutes to obtain light-burned dolomite emulsion;
[0026] 2) Introduce carbon dioxide into the light-burned dolomite lime emulsion, and control the gas flow of carbon dioxide to 40-50L h -1 , stirring while passing, the stirring speed is controlled as 150r / min, the pH value of the reaction mixture is detected while feeding carbon dioxide, and when the pH=6.89, the carbon dioxide is stopped, the filter residue calcium carbonate is removed, and the heavy magnesium aqueous solution is left to complete Calcium and magnesium are separated for the first time and most of the impurities and calcium ions are removed;
[0027] 3) Take the heavy magnesium aqueous solution, add absolute ethanol equivalent to 40% of the volume of the heavy magnesium aqueous solution, heat it in a water bath and control the temperature of the water bath to 80°C and pyrolyze it for 1 hour, then filter to ob...
Embodiment 2
[0033] 1) Pour 60g of light-burned dolomite powder into 1 liter of water at 55°C and digest it for 50 minutes to obtain light-burned dolomite emulsion;
[0034] 2) Introduce carbon dioxide into the light-burned dolomite lime emulsion, and control the gas flow of carbon dioxide to 70-80L h -1, stirring while passing, the stirring speed is controlled to be 160r / min, and the pH value of the reaction mixture is detected while feeding carbon dioxide, and when the pH=6.94, the carbon dioxide is stopped, the filter residue calcium carbonate is removed, and the heavy magnesium aqueous solution is left to complete Calcium and magnesium are separated for the first time and most of the impurities and calcium ions are removed.
[0035] 3) Take the heavy magnesium aqueous solution, add absolute ethanol equivalent to 30% of the volume of the heavy magnesium aqueous solution, heat it in a water bath and control the temperature of the water bath to 60°C and pyrolyze it for 1.5 hours, then fil...
Embodiment 3
[0041] 1) Pour 60g of light-burned dolomite powder into 1 liter of water at 65°C and digest it for 45 minutes to obtain light-burned dolomite emulsion;
[0042] 2) Introduce carbon dioxide into the light-burned dolomite lime emulsion, and control the gas flow of carbon dioxide to 60-70L h -1 , stirring while passing, the stirring speed is controlled as 150r / min, the pH value of the reaction mixture is detected while passing into carbon dioxide, and when the pH=6.70, the carbon dioxide is stopped, the filter residue calcium carbonate is removed, and the heavy magnesium aqueous solution is left to complete Calcium and magnesium are separated for the first time and most of the impurities and calcium ions are removed;
[0043] 3) Take the heavy magnesium aqueous solution, add absolute ethanol equivalent to 35% of the volume of the heavy magnesium aqueous solution, heat it in a water bath and control the temperature of the water bath to 50° C. After pyrolysis for 2 hours, filter to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com