A kind of non-steroidal anti-inflammatory tablet and preparation method thereof
A non-steroidal anti-inflammatory drug, non-steroidal anti-inflammatory technology, applied in the direction of anti-inflammatory agents, antipyretics, drug combinations, etc., can solve the problems of low temperature fusion, increased gastrointestinal stimulation, tablet sticking and punching, etc. , to achieve the effect of improving stability, superior performance, and improving dissolution and release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
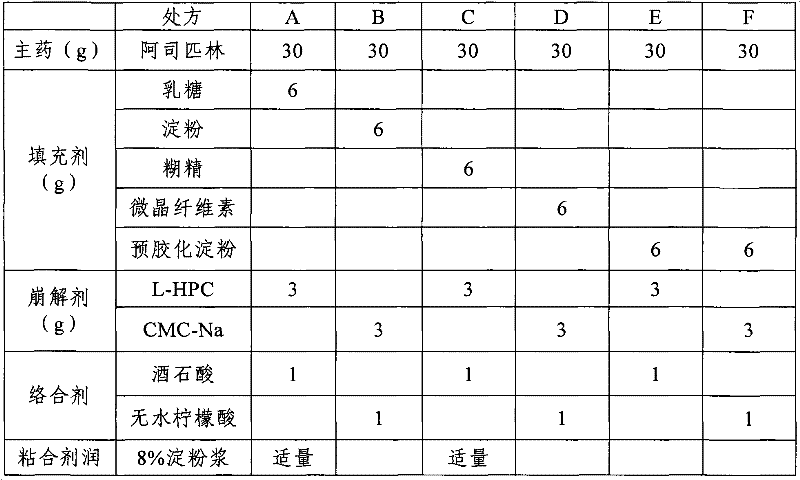
[0010] 1. Raw material and formula
[0011] Aspirin 0.3g
[0012] Pregelatinized starch 0.06g
[0013] Anhydrous citric acid 0.01g
[0014] Hydroxypropyl cellulose 0.03g
[0015] 8% starch slurry 0.001g
[0017] 2. Preparation method
[0018] Consists of the following steps:
[0019] (1) Pulverize each raw and auxiliary material, pass through a 80-100 mesh sieve, and weigh for subsequent use;
[0020] (2) Mix aspirin, pregelatinized starch, anhydrous citric acid, and hydroxypropyl cellulose evenly;
[0021] (3) make soft material with 8% starch slurry, granulate, dry;
[0022] (4) Add talcum powder, mix and press into tablets.
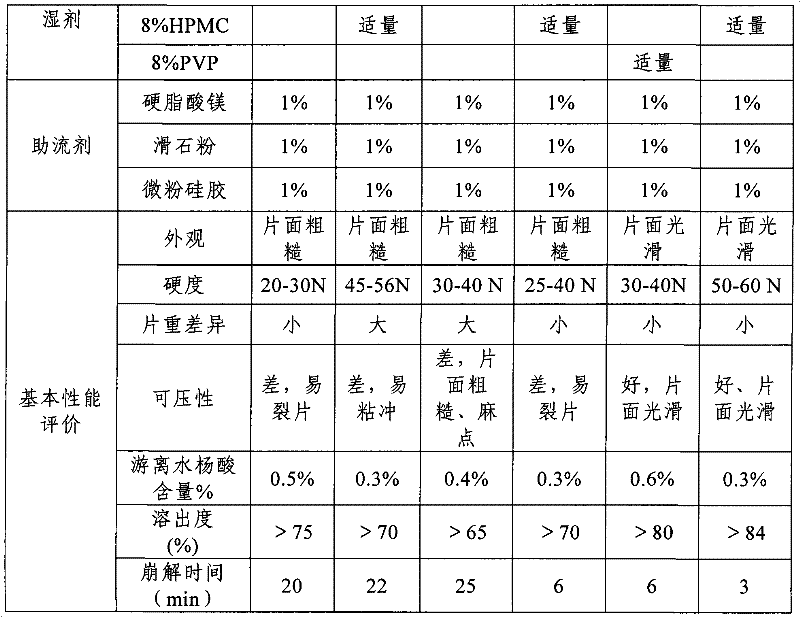
Embodiment 2
[0024] 1. Raw material and formula
[0025] Aspirin 0.3g
[0026] Pregelatinized starch 0.06g
[0027] Anhydrous citric acid 0.01g
[0028] Hydroxypropyl cellulose 0.03g
[0029] 5% hypromellose 0.001g
[0031] 2. Preparation method
[0032] Consists of the following steps:
[0033] (1) Pulverize each raw and auxiliary material, pass through a 80-100 mesh sieve, and weigh for subsequent use;
[0034] (2) Mix aspirin, pregelatinized starch, anhydrous citric acid, and hydroxypropyl cellulose evenly;
[0035] (3) make soft material with 5% hypromellose, granulate, dry;
[0036] (4) Add talcum powder, mix and press into tablets.
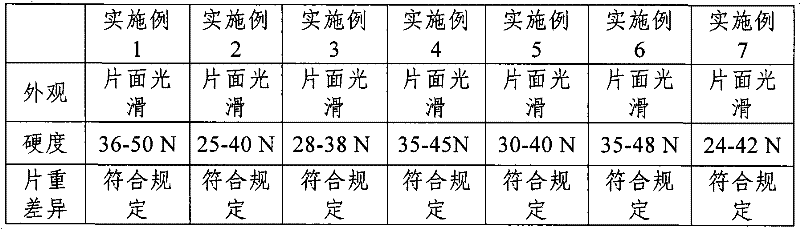
Embodiment 3
[0038] 1. Raw material and formula
[0039] Aspirin 0.3g
[0040] Pregelatinized starch 0.06g
[0041] Anhydrous citric acid 0.01g
[0042] Hydroxypropyl cellulose 0.03g
[0043] 8% polyvinylpyrrolidone 0.001g
[0044] Micropowder silica gel 0.004g
[0045] 2. Preparation method
[0046] Consists of the following steps:
[0047] (1) Pulverize each raw and auxiliary material, pass through a 80-100 mesh sieve, and weigh for subsequent use;
[0048] (2) Mix aspirin, pregelatinized starch, anhydrous citric acid, and hydroxypropyl cellulose evenly;
[0049] (3) make soft material with 8% polyvinylpyrrolidone, granulate and dry;
[0050] (4) Add micropowder silica gel to mix and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com