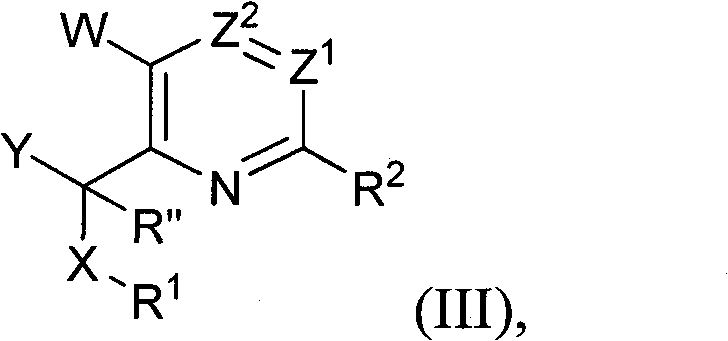
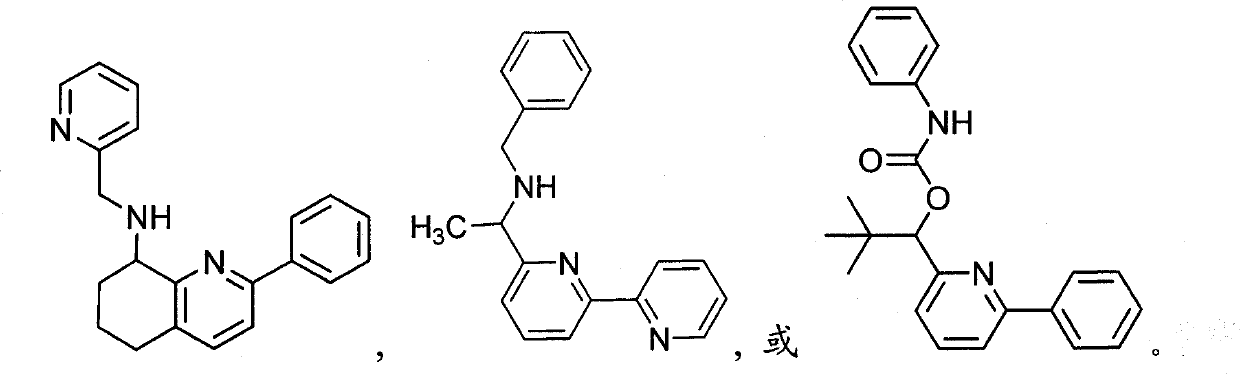
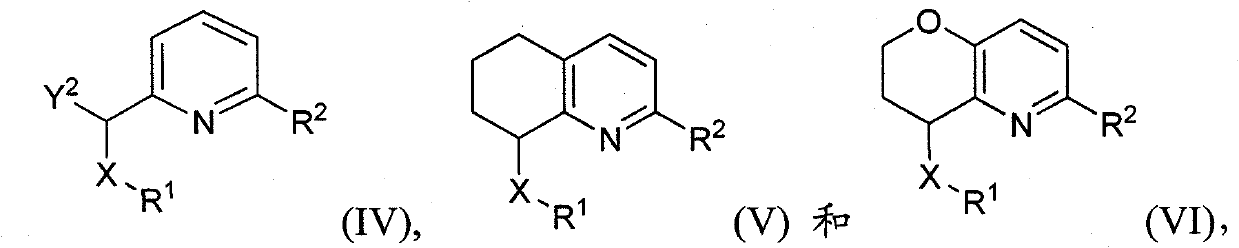
Pyridines, bicyclopyridines, and related analogs as sirtuin modulators
A technology of heterocycles and compounds, applied in the field of pyridine, bicyclic pyridine and related analogs as sirtuin regulators, can solve the problems of prolonging the lifespan of wild-type cells and reducing the
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0377] Example 1 Preparation of N-(1-(6-(3-(trifluoromethyl)phenyl)pyridin-2-yl)ethyl)pyrazine-2-carboxamide (compound 204):
[0378] Step 1. Synthesis of 1-(6-(3-(trifluoromethyl)phenyl)pyridin-2-yl)ethanone (2):
[0379]
[0380] 3-(Trifluoromethyl)phenylboronic acid (43; 500mg, 2.63mmol), 1-(6-bromopyridin-2-yl)ethanone (1; 438mg, 2.19mmol), Pd[Ph 3 P] 4 (100mg) and 2M K 2 CO 3 A mixture of aqueous solution (3 mL) in 25 mL toluene was stirred at 90°C for 1.5 hours. LC showed the reaction was complete. The solution was cooled, extracted with EtOAc (50 mL), washed with 25 mL of 2 mol / L NaOH(aq), 25 mL of brine, washed over anhydrous MgSO 4 Dried and concentrated. The resulting residue was purified by chromatography (EtOAc / petroleum ether = 1:30) to afford 1-(6-(3-(trifluoromethyl)phenyl)pyridin-2-yl)ethanone 2 as white Solid (538 mg, yield: 92%). Calculated MS(ESI): C 14 h 10 f 3 NO: 265.07; found value: 266 [M+H].
[0381] Step 2. Synthesis of 1-(6-(3-(trifluo...
Embodiment 2
[0391] Example 2 Preparation of N-2-pyrazinyl-2-{6-[3-(trifluoromethyl)phenyl]-2-pyridyl}propanamide (compound 214):
[0392] Synthesis of step 1.2-(2-pyridyl) ethyl propionate (7):
[0393]
[0394] The method detailed here is similar to that described in WO2005 / 051919. A hexane solution of n-butyllithium (24ml, 60mmol) was added to a solution of diisopropylamine (8.56ml, 60.0mmol) in tetrahydrofuran (20mL). The resulting solution was stirred at -78°C for 15 minutes. Ethyl 2-pyridylacetate (6; 3ml, 19.69mmol) was added, and the mixture was stirred at -78°C for 30 minutes, followed by the addition of iodomethane (6.15ml, 98mmol). The reaction mixture was stirred at -78°C for 15 minutes, then at room temperature for 3 hours. The reaction mixture was cooled in an ice bath, and 20 mL of water was added. It was extracted with EtOAc (3x50ml). The combined organics were washed with brine, dried over sodium sulfate, and concentrated to give 7 as a red oil. It was purified by...
Embodiment 3
[0411] Example 3 Preparation of N-({6-[3-(trifluoromethyl)phenyl]-2-pyridyl}methyl)benzamide (compound 208):
[0412] Step 1. Synthesis of 6-[3-(trifluoromethyl)phenyl]-2-pyridinecarbaldehyde (14):
[0413]
[0414] 6-Bromo-2-pyridinecarbaldehyde (13; 1 g, 5.38 mmol) was dissolved in toluene (50 mL), then [3-(trifluoromethyl)phenyl]boronic acid (1.123 g, 5.91 mmol), tetrakis( Triphenylphosphine) palladium (0) (tetrakis) (0.217 g, 0.188 mmol) and 2M potassium carbonate (aq) (6.45 mL, 12.90 mmol). The reaction mixture was stirred overnight at 90 °C. Water was added and the mixture was extracted with EtOAc (100ml). The organics were separated and the solvent was removed.
[0415] Purification by silica gel chromatography (0 to 20% gradient of ethyl acetate in hexanes) afforded the title compound 14 as a yellow oil (880 mg, yield: 32.6%). This product was used directly in the next step. Calculated MS(ESI): C 13 h 8 f 3 NO: 251.20; found value: 252.1 [M+H].
[0416] Syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com