A kind of dianhydride containing 1,2,2-trimethylcyclopentyl alicyclic structure and its preparation method
A technology of trimethylcyclopentyl ester and dianhydride, which is used in the preparation of polyimide film materials and the field of polyimide dianhydride monomers, can solve the difficulty of processing and molding, reduce light transmittance, insoluble, etc. problems, to achieve the effects of low manufacturing cost, simple preparation process, good transparency and heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
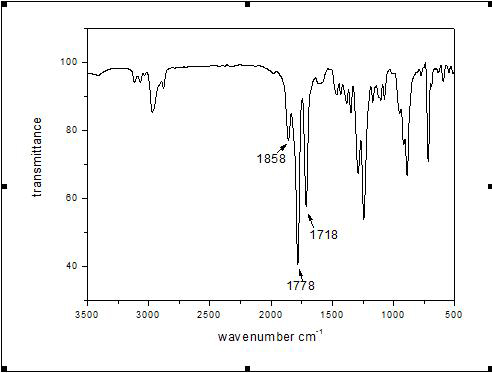
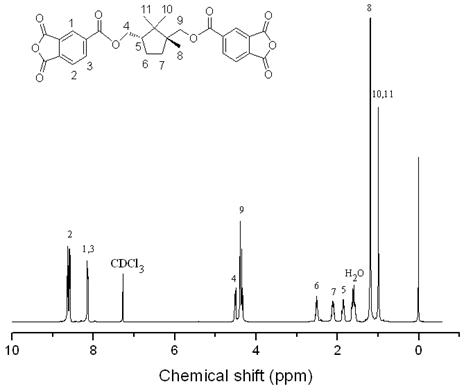
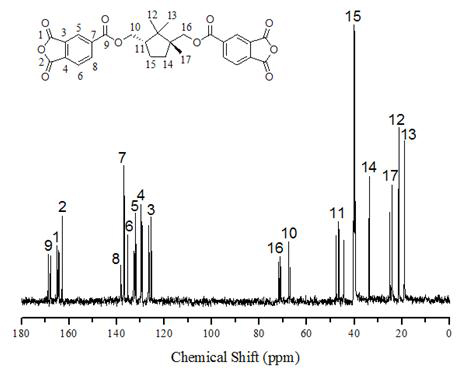
[0023] In a 1000mL round bottom flask, add 38g natural camphor containing [2,2,1] bicyclic structure, 2g ZnSO 4 ·7H 2 O, 170mL of distilled water and 360mL of concentrated nitric acid were refluxed at a temperature of 100-110°C for 30h, the resulting solution was cooled to room temperature, and the white solid diacid was obtained by suction filtration, which was purified and dried in vacuum with a yield of 33%. In a 100mL three-neck flask, add 2.0gLiAlH 4 , 3.0g white solid diacid and 70mL anhydrous ether, after reflux at room temperature for 5h, slowly add 8.5gNa 2 SO 4 10H 2 O for quenching, add 50mL CH after quenching 2 Cl 2 Wash, filter with suction, and filter the cake with 50mL CH 2 Cl 2 After washing and suction filtration, the filtrates were combined, and the filtrate was concentrated to obtain a white solid diol, which was purified and dried, with a yield of 50%. In a 250mL three-necked flask, add 7.98g of chlorinated trimellitic anhydride, 2.58g of white soli...
Embodiment 2-4
[0025] 2g FeSO 4 ·7H 2 O-170mL distilled water-360mL concentrated nitric acid system, 2g FeSO 4 ·7H 2 O-170mL distilled water-320mL fuming nitric acid, 2g FeSO 4 ·7H 2 O-170mL distilled water-360mL concentrated sulfuric acid system instead of 2g ZnSO 4 ·7H 2 O, 170mL distilled water and 360mL concentrated nitric acid system, the productive rate is 54%, 56% and 30% respectively. All the other reaction conditions are with specific embodiment 1.
Embodiment 5-6
[0027] The mixed solution of 70mL tetrahydrofuran, 30mL anhydrous ether and 40mL tetrahydrofuran was used instead of 70mL anhydrous ether, and the yields were 57% and 85.3%, respectively. All the other reaction conditions are with specific embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com