Application of a Catalyst in Alkaline Fuel Cell
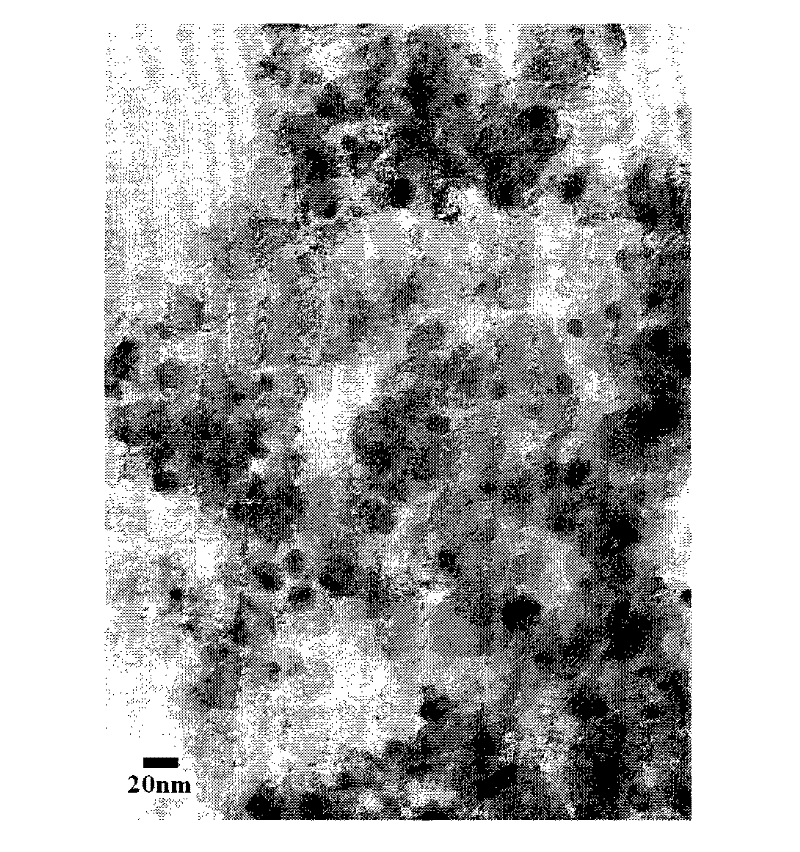
A technology of fuel cells and catalysts, applied in physical/chemical process catalysts, battery electrodes, circuits, etc., to achieve good application effects, small particles, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation and activity test of 29% Co-N / C catalyst:
[0030] Add 2.0mL of cobalt nitrate hexahydrate ethanol solution with a concentration of 10mgCo / mL and 80mg of imidazole into 50mL of ethanol, ultrasonically stir until uniform, and obtain mixed solution A; add 0.1g of XC-72 carbon powder into 50mL of ethanol, ultrasonically Mix evenly to obtain slurry B; mix the above slurry A with solution B, evaporate ethanol to dryness under stirring condition at 60°C, after drying, pass through N 2 , heat-treated at 600° C. for 2 hours, and cooled to room temperature to obtain a Co-N / C catalyst with an active component content of 29% and a N:Co molar ratio of 4.4:1.
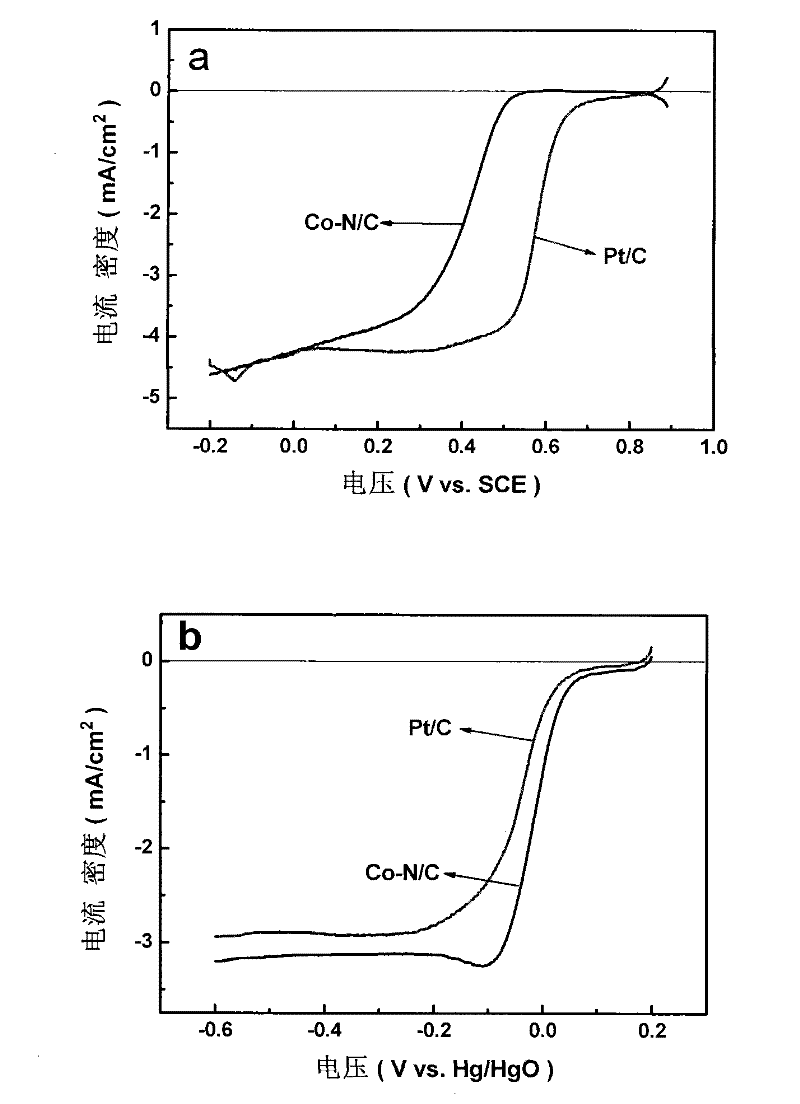
[0031] Electrochemical oxygen reduction (ORR) polarization curve tests were carried out on the catalyst prepared above and the commercial Pt / C comparison catalyst respectively, and the test conditions were as follows:
[0032] Cyclic voltammetry (CV) tests were performed on a CHI 600 electrochemical tester and a ...
Embodiment 2
[0038] Preparation and activity test of 10% Fe-N / C catalyst:
[0039] Add 1.33mL of ferrous chloride dihydrate isopropanol solution with a concentration of 5mgFe / mL and 55.2mg of hexamethylenediamine into 40mL of isopropanol, ultrasonically stir until uniform to obtain mixed solution A; mix 0.18g XC- Add 72 carbon powder into 60mL isopropanol, and mix evenly by ultrasonic to obtain slurry B; mix the above slurry A with solution B, and evaporate the isopropanol to dryness under stirring at 75°C. After drying, pass in NH 3 , heat-treated at 300°C for 1 hour, and cooled to room temperature to obtain an Fe-N / C catalyst with an active component content of 10% and a N:Fe molar ratio of 8:1.
[0040] Electrochemical oxygen reduction (ORR) polarization curve tests were carried out on the catalyst prepared above and the commercial Pt / C comparison catalyst respectively, and the test conditions were as follows:
[0041] Cyclic voltammetry (CV) tests were performed on a CHI 600 electroch...
Embodiment 3
[0047] Preparation and activity test of 80% Ni-N / C catalyst:
[0048] Add 5.93mL of ethanol solution of nickel oxalate dihydrate with a concentration of 8mgNi / mL and 747.7mg of aniline into 80mL of ethanol, ultrasonically stir until uniform, and obtain mixed solution A; add 0.04g of BP2000 carbon powder into 20mL of ethanol, and ultrasonically mix uniform to obtain slurry B; mix the above slurry A with solution B, evaporate ethanol to dryness at 60°C under stirring condition, after drying, pass through N 2 , heat-treated at 750°C for 2.5 hours, and cooled to room temperature to obtain a Ni-N / C catalyst with an active component content of 80% and a N:Ni molar ratio of 10:1.
[0049] Electrochemical oxygen reduction (ORR) polarization curve tests were carried out on the catalyst prepared above and the commercial Pt / C comparison catalyst respectively, and the test conditions were as follows:
[0050] Cyclic voltammetry (CV) tests were performed on a CHI 600 electrochemical teste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com