Method for preparing epoxiconazole intermediate
The technology of an intermediate and epoxiconazole, which is applied in the field of preparation of epoxiconazole intermediates, can solve the problems of low content and yield, complicated operation and high cost, and achieves high content and yield, simple process and less three wastes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
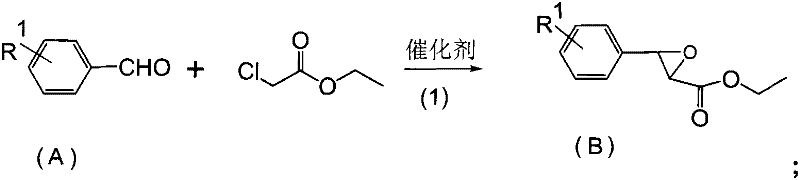
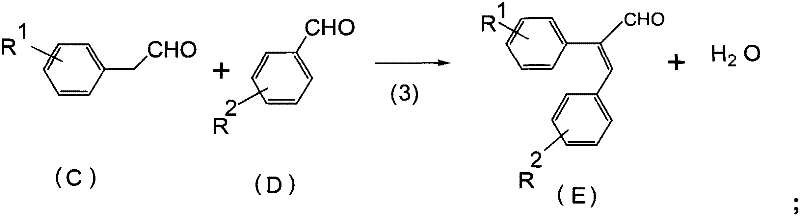
[0025] This embodiment provides a preparation method of 2-(4-fluorophenyl)-3-(2-chlorophenyl)-propene, which comprises the following steps:
[0026] (1), the synthesis of epoxy ester intermediate
[0027] Add 32g of ethanol in the reaction flask, dissolve 8.16g of sodium ethylate (0.12mol) in ethanol, add dropwise 12.4g of p-fluorobenzaldehyde (0.1mol) and 12.3g of ethyl chloroacetate (0.1004mol) in the system For the mixture, keep the temperature of the system at 10°C to 15°C during the dropwise addition process. After the dropwise addition, raise the temperature to 30°C and keep it warm for 3.5 hours. After the reaction is complete, remove ethanol, add water, and separate to obtain 20.5g of epoxy ester intermediate with a content of 94.3%. The rate is 92%.
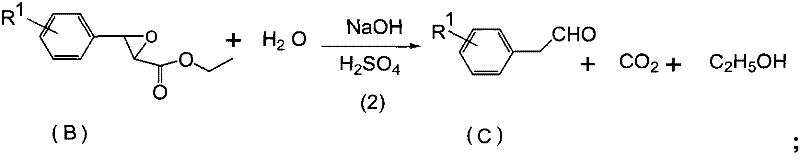
[0028] (2), the synthesis of p-fluorophenylacetaldehyde
[0029] Add the 20.5g epoxy ester intermediate (content 94.3%, 0.092mol) that step (1) obtains in reaction bottle, be warming up to 40 ℃, add the sodium hydroxid...
Embodiment 2
[0035] This embodiment provides a preparation method of 2-(4-methoxyphenyl)-3-(2-chlorophenyl)-propene, which comprises the following steps:
[0036] (1), the synthesis of epoxy ester intermediate
[0037] Add 32g of ethanol and 6.48g of sodium methylate (0.12mol) in the reaction flask, add dropwise the mixture of 13.4g p-methoxybenzaldehyde (0.1mol) and 12.3g of ethyl chloroacetate (0.1004mol) at 10°C, dropwise After the temperature was raised to 30°C for 2 hours, ethanol was removed, and water was added to separate the mixture to obtain 21.7 g of an epoxy ester intermediate with a content of 90.3% and a yield of 89%.
[0038] (2), the synthesis of p-methoxyphenylacetaldehyde
[0039] Add the 21.7g epoxy ester intermediate (content 90.3%, 0.0894mol) that step (1) obtains in the reaction bottle, 35.6g 20% sodium hydroxide aqueous solution (sodium hydroxide 0.178mol), react 2h at 40 ℃ , heated up to 90°C, added 152.4g of 20% dilute sulfuric acid (0.311mol of sulfuric acid),...
Embodiment 3
[0045] This embodiment provides a preparation method of 2-(4-fluorophenyl)-3-(4-chlorophenyl)-propene, which comprises the following steps:
[0046] (1), the synthesis of epoxy ester intermediate
[0047] Add 32g of methanol and 8.16g of sodium ethylate (0.12mol) in the reaction flask, add dropwise a mixture of 12.4g of p-fluorobenzaldehyde (0.1mol) and 12.3g of ethyl chloroacetate (0.1004mol) at 10°C, and the reaction ends Methanol was added to water layer to obtain 20.7 g of an epoxy ester intermediate with a content of 92.3% and a yield of 91.2%.
[0048] (2), the synthesis of p-fluorophenylacetaldehyde
[0049] Add the 20.7g epoxy ester intermediate (content 92.3%, 0.09mol) that step (1) obtains in reaction bottle, add the sodium hydroxide aqueous solution (sodium hydroxide 0.227mol) of 45.4g 20wt% at 40 ℃, end of reaction Raise the temperature to 90°C, add 154.4g of 20wt% dilute sulfuric acid (0.315mol of sulfuric acid), cool down to 30°C after 1h, and separate to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com