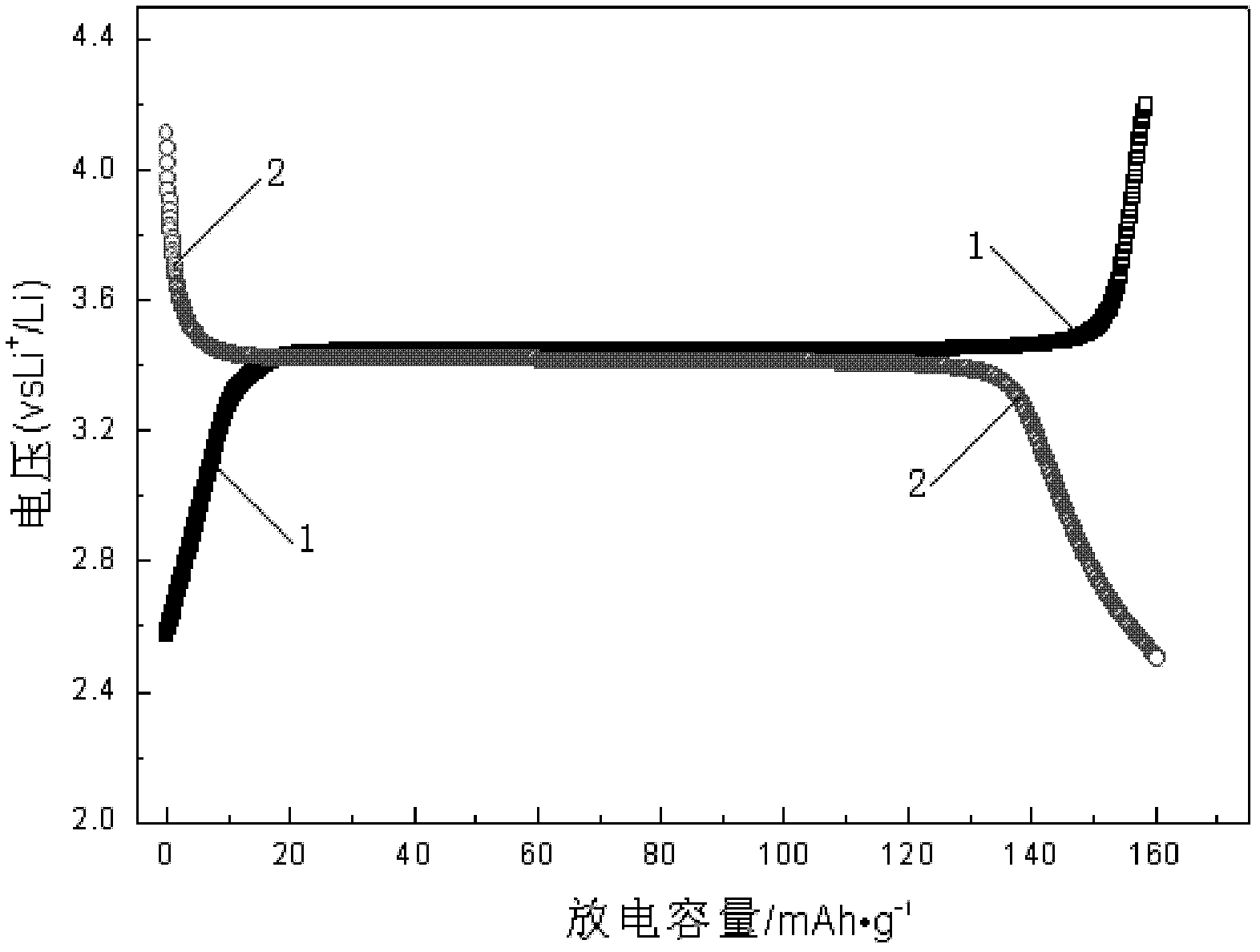
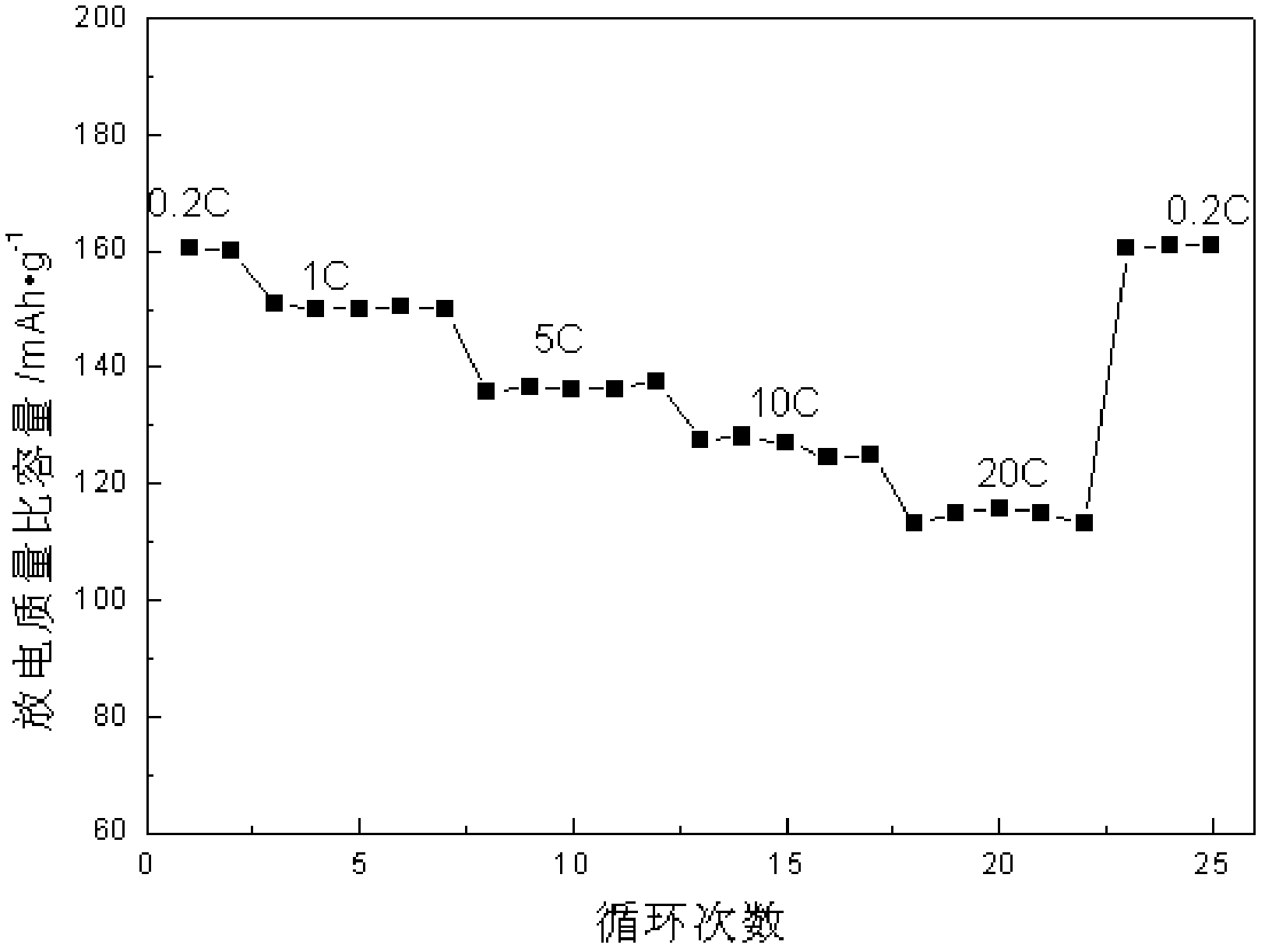
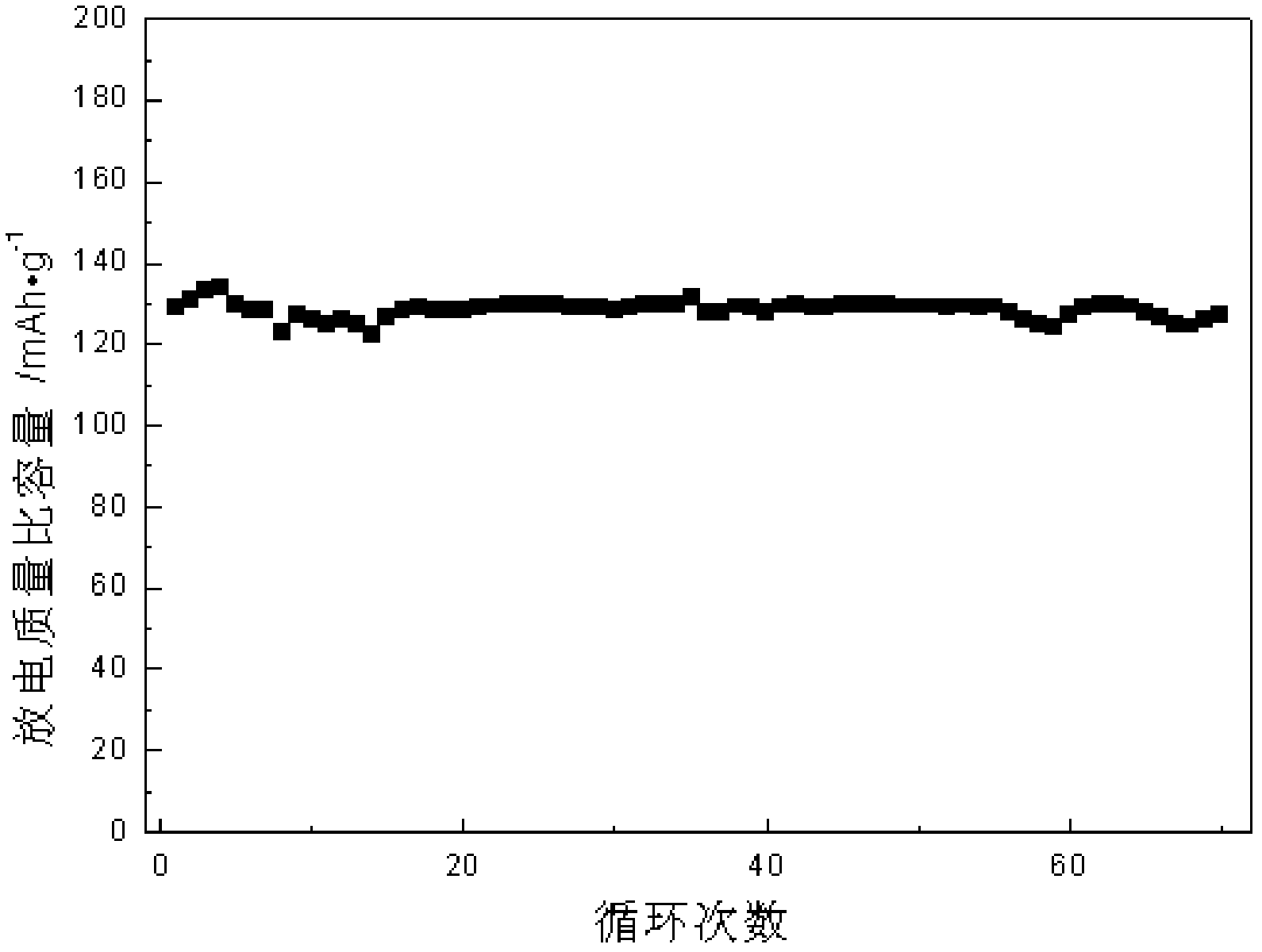
Multi-layer graphene/lithium iron phosphate intercalated composite material, preparation method thereof, and lithium ion battery adopting multi-layer grapheme/lithium iron phosphate intercalated composite material as anode material
A multi-layer graphene, lithium iron phosphate technology, applied in secondary batteries, battery electrodes, circuits, etc., can solve the problems of poor high-rate charge-discharge performance and poor conductivity, and achieve good conductivity, increased surface area, and reduced interface. Effect of Current Density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0014] Embodiment 1: This embodiment is a multilayer graphene / lithium iron phosphate intercalation composite material, which adopts multilayer graphene, ferric salt, phosphorus source compound, lithium source compound and organic small molecule carbon source The composite precursor is prepared by the rheological phase method, and then the composite precursor is sintered, wherein the molar ratio of Fe in the ferric salt to P in the phosphorus source compound is 1:1, Li in the lithium source compound and P in the phosphorus source The molar ratio of P in the compound is 1 to 1.1:1, the mass ratio of the organic small molecule carbon source to the theoretical yield of lithium iron phosphate is 0.4 to 0.8:1, and the mass ratio of the multilayer graphene to the theoretical yield of lithium iron phosphate is 0.005 ~0.3:1.
[0015] In this embodiment, the sandwich structure of the multilayer graphene / lithium iron phosphate intercalation composite material plays a positive role in imp...
specific Embodiment approach 2
[0017] Specific embodiment two: the difference between this embodiment and specific embodiment one is that the ferric salt is ferric nitrate or ferric chloride, the phosphorus source compound is ammonium dihydrogen phosphate or ammonium monohydrogen phosphate, and the lithium source The compound is one or a mixture of lithium nitrate, lithium carbonate and lithium hydroxide, and the organic small molecule carbon source is sucrose and / or glucose. Other parameters are the same as in the first embodiment.
[0018] In the present embodiment, when the lithium source compound is a mixture, it is mixed in an arbitrary ratio. When the organic small molecule carbon source is a mixture of the two, sucrose C 12 h 22 o 11 with glucose C 6 h 12 o 6 The molar ratio of the mixture is 1:2-4.
specific Embodiment approach 3
[0019] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the mass ratio of the organic small molecule carbon source to the theoretical yield of lithium iron phosphate is 0.5-0.7:1. Other parameters are the same as those in Embodiment 1 or Embodiment 2.
[0020] In this embodiment, the optimal mass ratio of the organic small molecule carbon source to the theoretical yield of lithium iron phosphate is 0.6:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com