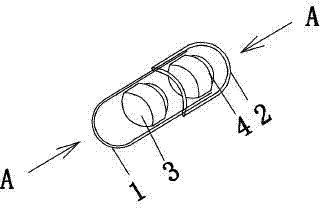
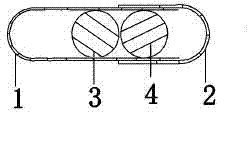
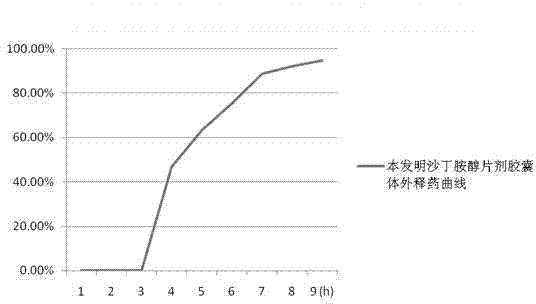
Levalbuterol hydrochloride oral controlled release tablet capsule and preparation method thereof
A technology for levosalbuterol hydrochloride and controlled-release tablets, which is applied in the field of medicine, can solve the problems of innocence, long preparation period, complicated process and the like, and achieves the effects of convenient treatment, short preparation period and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1: the preparation of levosalbutamol hydrochloride pulse tablet
[0085] (1) Preparation of pulsed core
[0086] Levalbuterol Hydrochloride 100g
[0087] Microcrystalline Cellulose KG-801 40g
[0088] Microcrystalline Fiber PH-302 32 g
[0089] Oligomeric Hydroxypropyl Cellulose 40g
[0090] Compressible starch 80g
[0092] Take by weighing main ingredient, microcrystalline cellulose, oligomeric hydroxypropyl cellulose, compressible starch, magnesium stearate and mix uniformly by prescription quantity, compress tablet, obtain the tablet core of 10mg / tablet.
[0093] (2) Preparation of pulse-coated tablets
[0094] Coat the tablet core with 160g of acrylic resin RS, 100g of acrylic resin L100, 10g of triethyl citrate, 30g of talcum powder, and 1000ml of absolute ethanol in a coating pan.
Embodiment 2
[0095] Embodiment 2: Preparation of Levosalbutamol Hydrochloride Sustained-release Tablets
[0096] (1) Preparation of sustained-release tablet cores
[0097] Levalbuterol Hydrochloride 100g
[0098] Lactose 100g
[0099] Microcrystalline Fiber PH-302 30g
[0100] Oligomeric Hydroxypropyl Cellulose 45g
[0101] Polyvinylpyrrolidone (K30) 15g
[0104] 75% ethanol appropriate amount
[0105] Dissolve polyvinylpyrrolidone in an appropriate amount of 75% ethanol, add the prescribed amount of drugs, lactose, microcrystalline fiber PH-302, and soft materials made of oligomeric hydroxypropyl cellulose, pass through a 40-mesh sieve, and ventilate and dry at 60°C. Sieve through a 60-mesh sieve for granulation, add an appropriate amount of magnesium stearate, mix evenly, and press into tablets to obtain a tablet core of 10 mg / tablet.
[0106](2) Preparation of sustained-release coated tablets
[0107] Coat the tablet core w...
Embodiment 3
[0108] Example 3: Capsule filling
[0109] Take No. 0 empty gelatin capsules, place them in a capsule filling machine, fill the above-mentioned pulse tablets and sustained-release tablets in each hollow capsule in turn, fill each capsule with one tablet with different drug-release properties, and pack it to obtain this product. Invention of tablet capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com