Resin ball capable of preferential adsorption of aromatic hydrocarbon component of hydrocarbon mixture, and preparation method thereof
A technology for hydrocarbon mixtures and resin balls, which is applied in the field of resin balls that can selectively adsorb aromatic components in hydrocarbon mixtures and its preparation, and can solve the problem of small adsorption capacity of adsorbents and the inability to use solvent oils with high aromatic content, etc. problems, to achieve the effect of simple operation, cost reduction and favorable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
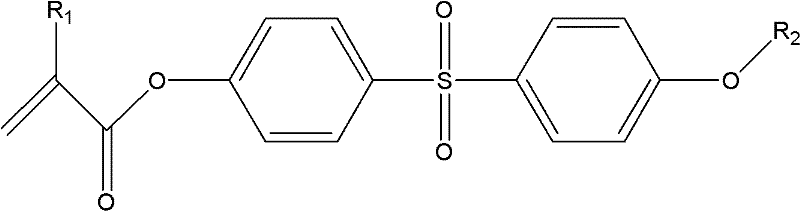
[0053] Embodiment 1: the synthesis of SDPDMA
[0054] Add 5.01g (20mmol) of SDP, 5.56mL (40mmol) of triethylamine and 20mL of dimethylformamide into a 100mL two-necked flask, mix well and place in an ice-salt bath, slowly dropwise add 5.81 A mixture of mL (60mmol) methacryloyl chloride and 25mL dimethylformamide. After the dropping, the solid was filtered out, and the obtained filtrate was dropped into 500 mL of distilled water drop by drop, and the precipitate was precipitated by stirring. After suction filtration, it was dried in a vacuum oven at room temperature to obtain a crude product, which was separated and purified by column chromatography.
[0055] 1 H NMR (500MHz, CDCl 3 , TMS): δ (ppm) 7.98 (4H, d, -Ph), 7.28 (4H, d, -Ph), 6.35 (2H, s, =CH 2 ), 5.80 (2H, s, = CH 2 ), 2.04 (6H, s, -CH 3 ).IR(KBr, cm -1 ): 1742 (C=O), 2924 (-CH 3 ), 1635 (C=C), 1323-1290 (=SO 2 ). It shows that SDPDMA has been prepared.
Embodiment 2
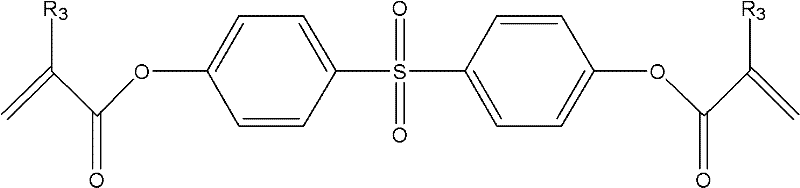
[0056] Embodiment 2: the synthesis of SDPMA
[0057] Add 5.01g (20mmol) of SDP, 2.08mL (15mmol) of triethylamine and 20mL of dimethylformamide into a 50mL two-necked flask. A mixture of mL (15 mmol) methacryloyl chloride and 14.5 mL dimethylformamide. After the dropping, the solid was filtered out, and the obtained filtrate was dropped into 400 mL of distilled water drop by drop, and the precipitate was precipitated by stirring. After suction filtration, it was dried in a vacuum oven at room temperature to obtain a crude product, which was separated and purified by column chromatography.
[0058] 1 H NMR (300MHz, CDCl 3 , TMS): δ (ppm) 7.93 (2H, d, -Ph), 7.76 (2H, d, -Ph), 7.25 (2H, d, -Ph), 6.85 (2H, d, -Ph), 6.51 ( 1H, s, -OH), 6.36 (1H, s, =CH 2 ), 5.81 (1H, s, =CH 2 ), 2.04 (3H, s, -CH 3 ).IR(KBr, cm -1 ): 1738 (C=O), 2927 (-CH 3 ), 1636 (C=C), 1315-1289 (=SO 2 ). It indicated that SDPMA was prepared.
Embodiment 3
[0059] Embodiment 3: Synthesis of resin balls with different degrees of crosslinking
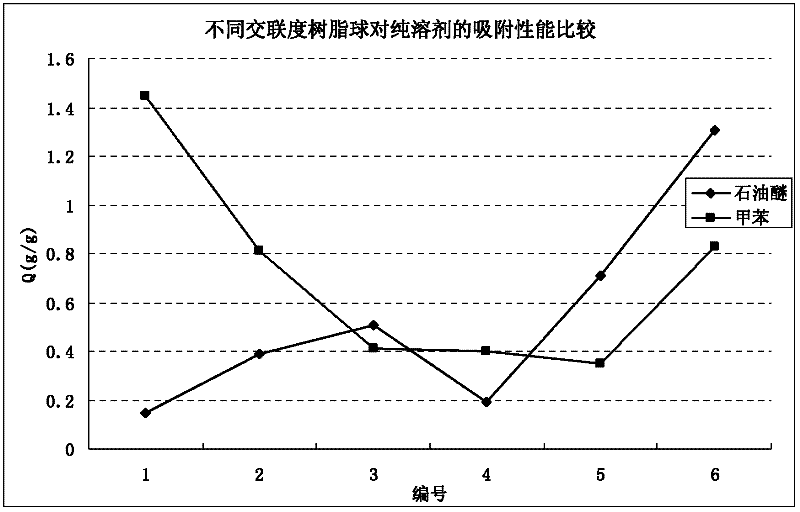
[0060] According to the formula in Table 1, after mixing the oil phase and the water phase evenly, pour them into the N 2 Into the three-necked flask of the introduction tube, reflux condenser, and mechanical stirrer, pass N 2 After 0.5h, put it into a constant temperature water bath preheated to 70°C, react at 225rpm for 1.5h, then raise the temperature to 85°C for 4h, and continue to raise the temperature to 90°C for 5h. After the reaction, the resin microspheres were filtered, extracted with dichloromethane for 24 hours, and dried to collect resin spheres with particle diameters in the range of 0.45-2 mm for future use. For the adsorption performance of resin balls on toluene and petroleum ether, see figure 1 (Note: The adsorption condition is 48h at room temperature).
[0061] Table 1: Raw material ratio of resin balls with different degrees of crosslinking
[0062]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com