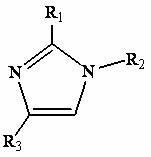
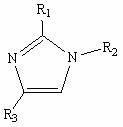
Production process of imidazole ionic liquid
A technology of ionic liquid and production process, which is applied in the field of preparation, can solve the problems of inconvenient transfer and use, unsuitable for large-scale production, and reduction of reactant substances, etc., and achieves short reaction time, convenient washing and separation operation, and small solid product particles Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Synthesis of Ionic Liquid: 1-Ethyl-3-Methylimidazole Bromide
[0016] Mix 7 kg of N-methylimidazole and 37.22 kg of ethyl bromide (ratio of the two substances is 1:4) in a reaction kettle, stir and react at 30°C for 6 hours, and stand to separate the excess reactants in the upper layer. The solid product was washed three times with ethyl acetate, each time it was placed in a centrifuge to separate the detergent, and finally dried in vacuum. The yield was 91%. The melting point of the solid product measured by a melting point instrument is 72-76° C., the purity of the product by liquid chromatography analysis is greater than 99%, and the water content of the product is less than 0.2% as measured by a Karl Fischer instrument.
[0017] The NMR spectrum of 1-ethyl-3-methylimidazole bromide is: 1 H NMR (DMSO-D 6 ): 9.41 (s, 1H, CH), 7.78 (t, 1H, CH), 7.69 (t, 1H, CH), 4.18-4.15 (q, 2H, CH 2 ), 3.28(s, 3H, CH 3 ), 1.37-1.35 (t, 3H, CH 3 ). It is proved that the obtained...
Embodiment 2
[0019] Synthesis of Ionic Liquid: 1-Ethyl-3-Methylimidazole Bromide
[0020] Mix 6 kg of N-methylimidazole and 39.88 kg of bromoethane (ratio of the two substances is 1:5) in a reaction kettle, stir and react at 20°C for 12 hours, and stand to separate the excess reactant in the upper layer. The solid product was washed three times with ethyl acetate, each time it was placed in a centrifuge to separate the detergent, and finally vacuum-dried, the yield was 89%. The melting point of the solid product measured by a melting point instrument is 72-76° C., the purity of the product by liquid chromatography analysis is greater than 99%, and the water content of the product is less than 0.2% as measured by a Karl Fischer instrument.
[0021] Nuclear magnetic spectrum is as embodiment 1.
Embodiment 3
[0023] Synthesis of Ionic Liquid: 1-Butyl-3-methylimidazole Bromide
[0024] Mix 8 kg of N-methylimidazole and 40.10 kg of bromobutane (ratio of the two substances is 1:3) in a reaction kettle, stir and react at 50°C for 36 hours, stand still to separate the excess reactant in the upper layer, and put The solid product was washed three times with ethyl acetate, each time it was placed in a centrifuge to separate the detergent, and finally vacuum-dried, the yield was 89%. The melting point of the solid product was 70-75°C as measured by the melting point instrument, and the product was analyzed by liquid chromatography The purity is greater than 98.5%, and the water content of the product measured by the Karl Fischer instrument is less than 0.2%.
[0025] The NMR spectrum of 1-butyl-3-methylimidazole bromide is: 1 H NMR (DMSO-D 6 ): 9.44 (s, 1H, CH), 7.89 (t, 1H, CH), 7.80 (t, 1H, CH), 4.19-4.15 (t, 2H, CH 2 ), 3.86(s, 3H, CH 3 ), 1.72-1.69 (m, 2H, CH 2 ), 1.18-1.69 (m, 2H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com