Copolymer of olefin and conjugated diene, and process for producing same
一种共轭二烯、共聚物的技术,应用在烯烃与共轭二烯的共聚物领域,能够解决乙烯基量不充分、机械特性不充分、耐气候性、耐热性·耐臭氧性下降等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
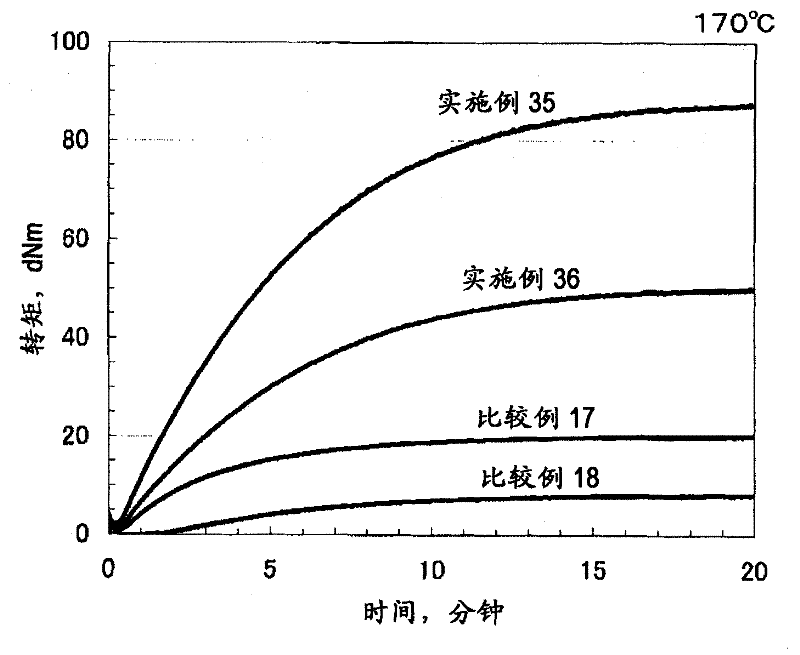
Examples
preparation example Construction
[0146] [Preparation method of copolymer]
[0147] The production method of the copolymer of the present invention is a method for producing the above-mentioned copolymer. The method for producing a copolymer of the present invention is the method for producing a copolymer as described above, characterized in that at least ethylene and conjugated diene copolymerization.
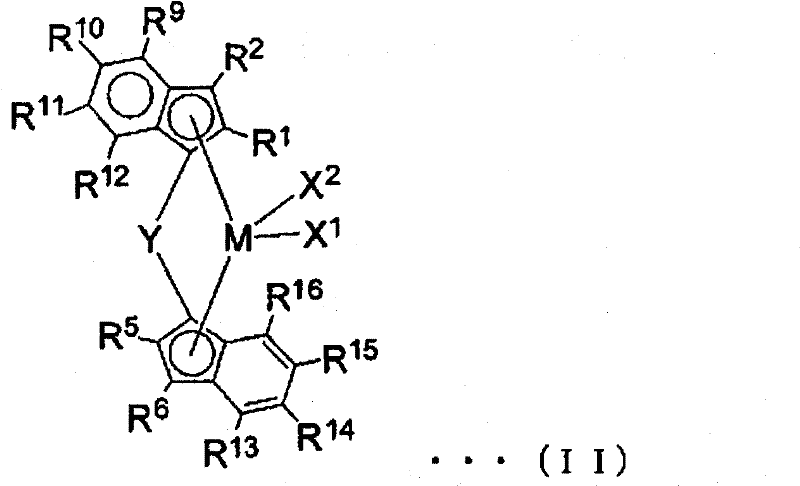
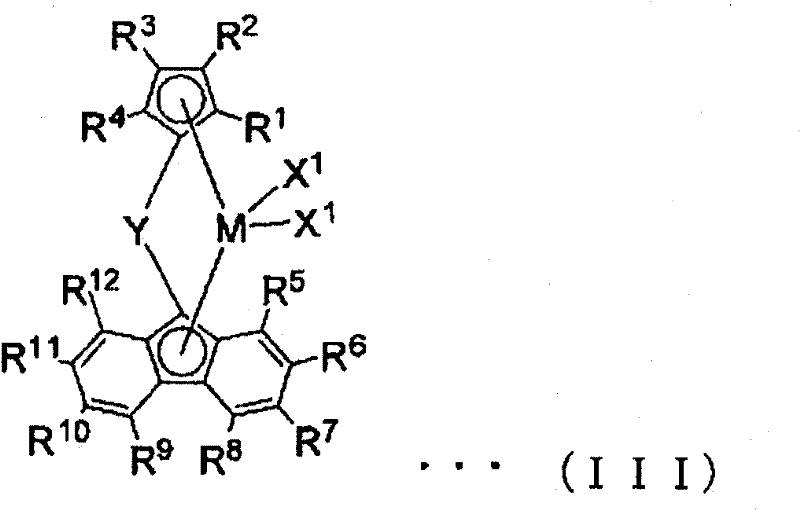
[0148] (A): A transition metal compound having a transition metal atom selected from Groups IB, IIIB to VIIIB of the Periodic Table of Elements
[0149] (B): at least one compound selected from the following compounds, which are
[0150] (B-1) organometallic compounds,
[0151] (B-2) organoaluminum oxy compounds, and
[0152] (B-3) A compound that reacts with a transition metal compound (A) to form an ion pair
[0153] In addition, said (A) is also described as a component (A), and said (B) is also described as a component (B).
[0154] It should be noted that the copolymer of the present invention is a co...
Embodiment 1
[0869] Toluene and triisobutylaluminum (also denoted as iBu3Al) 0.2mmol, at 30°C, with 1.0kg / cm 2 The 1,3-butadiene in G saturates the liquid and gas phases. The above mixture was heated to 40°C, so that the internal pressure of the reactor was 1.1kg / cm 2 G, and then pressurized by ethylene gas to saturate the liquid phase and gas phase, so that the internal pressure of the reactor is 2.0kg / cm 2 ·G.
[0870] After that, 0.2 mL of dimethylsilyl {1-(2-methyl-4,5-benzindenyl)} synthesized by referring to the method described in J. Organomet. Chem. 2003, (688), 153 was added (2,7-di-tert-butylfluorenyl) toluene solution (10mmol / L) of zirconium dichloride (complex 1), then, add triphenylcarbenium tetrakis(pentafluoro Phenyl) borate (also denoted as Ph 3 CB(C 6 f 5 ) 4 ) in toluene solution (4mmol / L), start to polymerize. The toluene to be charged was finally adjusted to 5 mL in total. Continuously supply ethylene gas to keep the total pressure at 2.0kg / cm 2 • G, after al...
Embodiment 2
[0874] Toluene and dry methylalumoxane (Albemarle company The product obtained by distilling off the impurity in methylaluminoxane (20% by weight), that is, trimethylaluminum; also referred to as DMAO) in toluene solution (Al=1.32M), the amount of methylaluminoxane Converted to aluminum is 1.5mmol, at 30°C, use 1.0kg / cm 2 The 1,3-butadiene in G saturates the liquid and gas phases. The above mixture was heated to 40°C, so that the internal pressure of the reactor was 1.1kg / cm 2 G, and then pressurized by ethylene gas to saturate the liquid phase and gas phase, so that the internal pressure of the reactor is 9.0kg / cm 2 · G,.
[0875] After that, 0.2 mL of rac-dimethylsilyl-bis[1-(2-methyl-4-phenylindenyl)]dichloride synthesized according to the method described in Organometallics 1994, 13, p.954 was added Zirconium (rac-Dimethylsilyl-bis[1-(2-methyl-4-phenylindenyl)]zirconium dichloride) (complex 2) in toluene solution (2.5mmol / L), start polymerization. Toluene was added to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com